Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Zasocitinib (TAK-279), an oral, allosteric, selective, tyrosine kinase 2 (TYK2) inhibitor in late-stage clinical development for the treatment of immune-mediated inflammatory diseases including psoriatic arthritis, was developed using AI-assisted compound design to create a next-generation TYK2 inhibitor with >1.3 millionfold greater affinity for TYK2 than Janus kinase (JAK)1/2/3.1
Methods: Whole blood culture from 12–13 healthy volunteers was used to characterize concentration–response (defined as percentage inhibition) curves for TYK2- (IL-12/18 IFN-γ) and JAK1/3- (IL-2 pSTAT5) dependent pathways for zasocitinib and licensed TYK2 and JAK inhibitors. Statistical modeling determined the relationship between concentration and percentage inhibition, and half-maximal inhibitory concentration (IC50) values were estimated. Plasma concentrations at clinically relevant/approved doses for each drug were simulated using population pharmacokinetic models from the literature. The time over which plasma concentrations exceeded the IC50, as well as the percentage daily inhibition, were compared between drugs for each pathway.
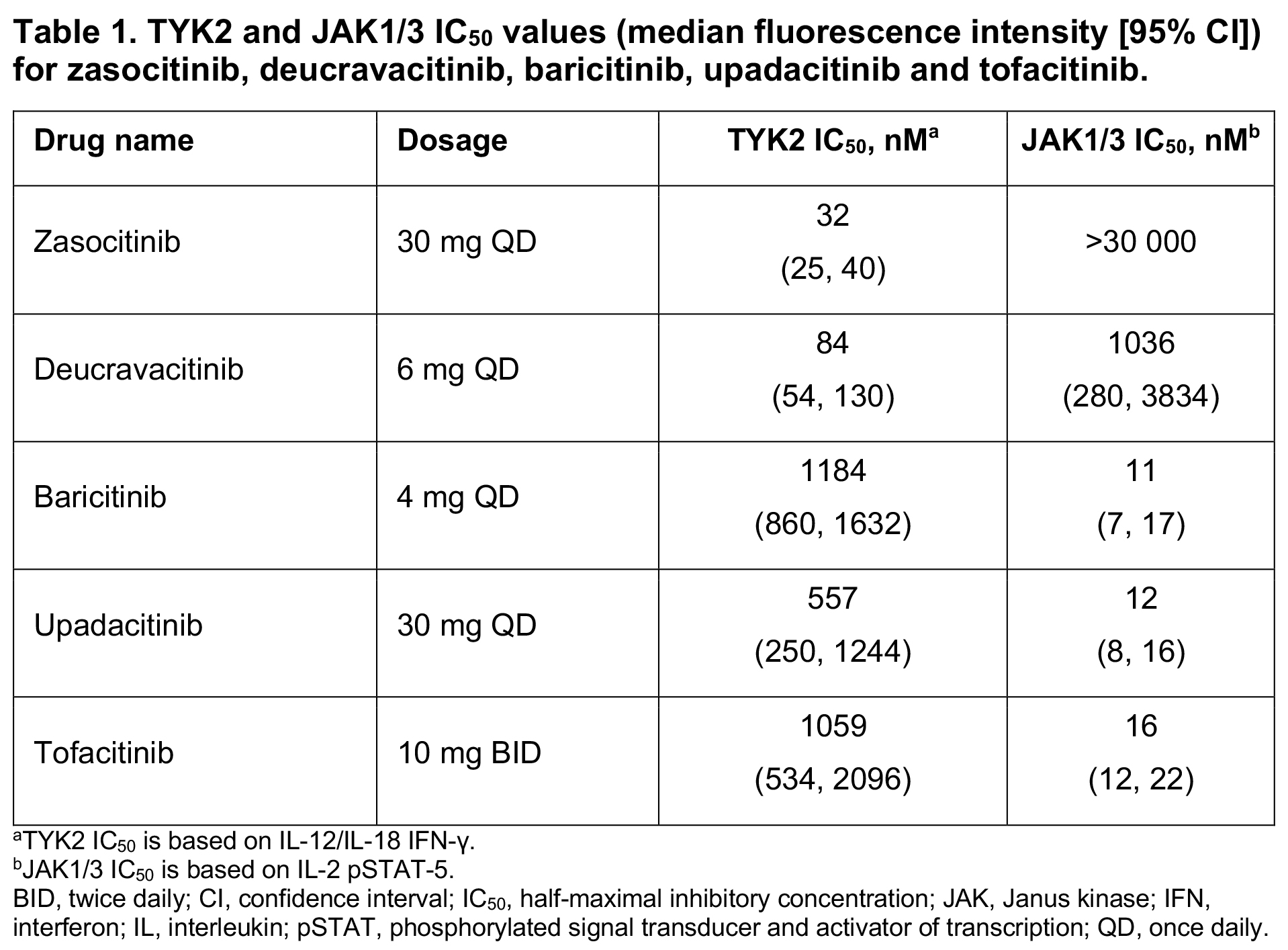
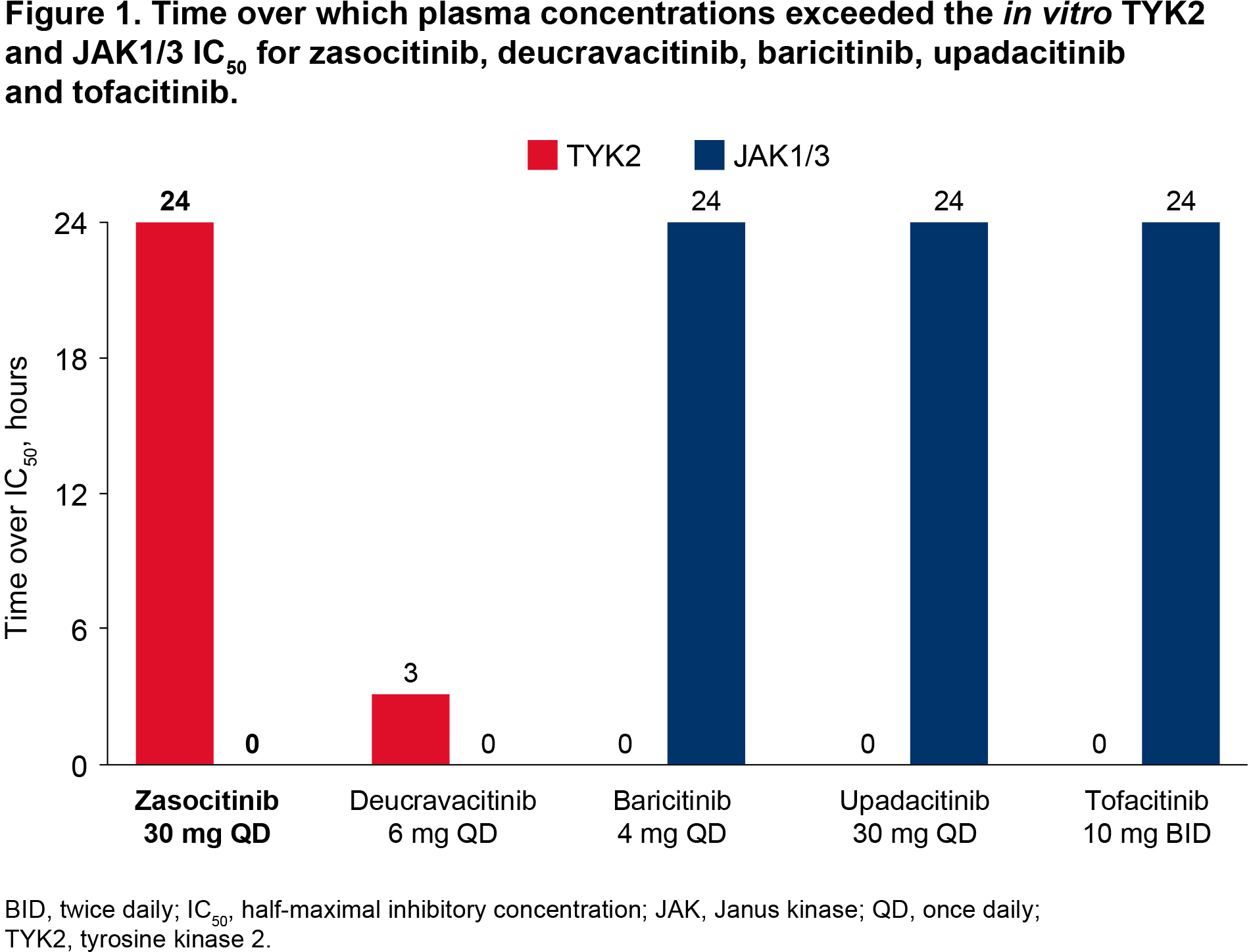
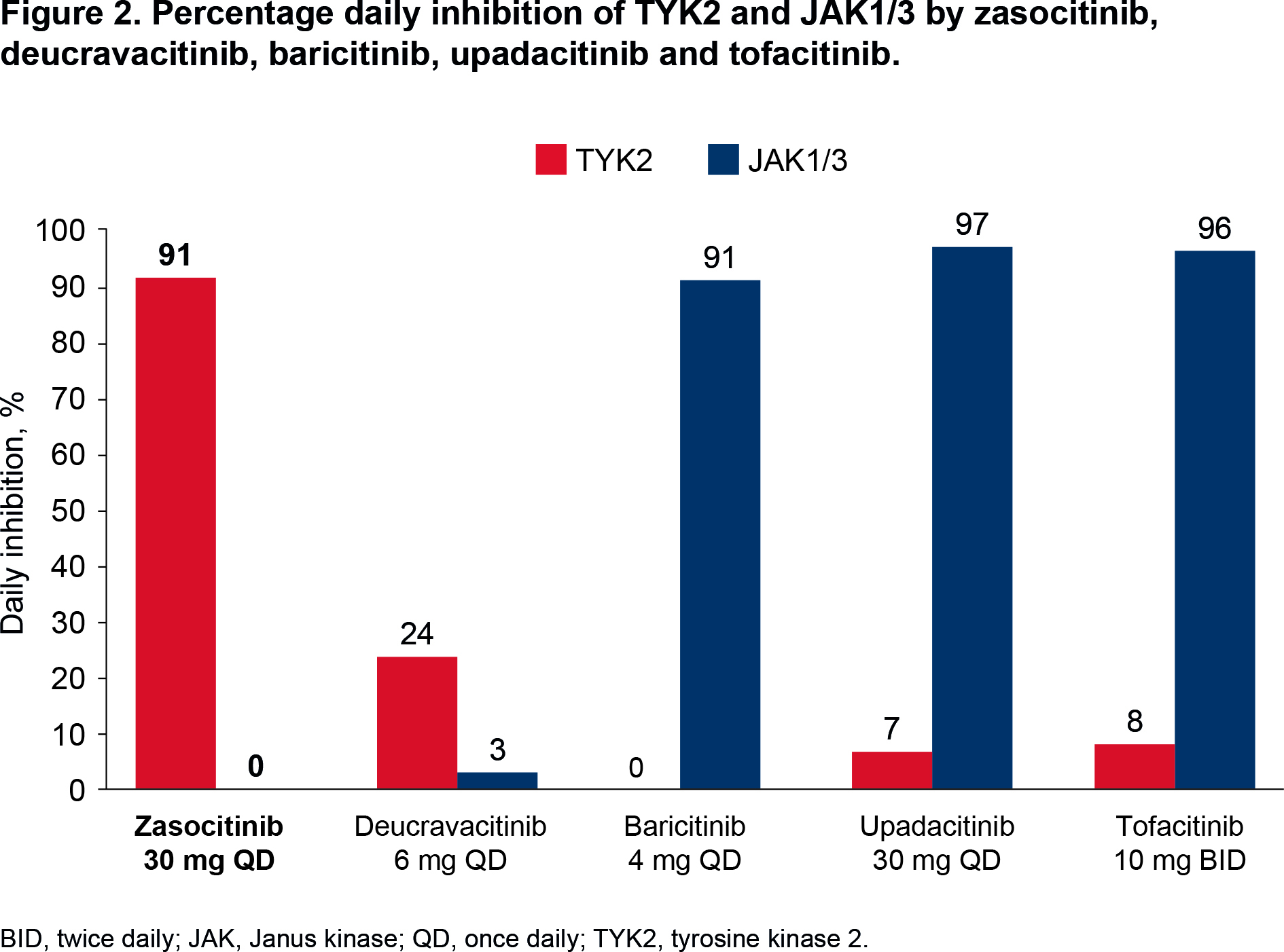
Results: Simulated plasma concentrations remained above the TYK2 IC50 for 24 hours for zasocitinib 30 mg QD versus 3 hours for deucravacitinib 6 mg QD, and 0 hours for baricitinib 4 mg once daily [QD], tofacitinib 10 mg twice daily [BID] and upadacitinib 30 mg QD (Table 1, Figure 1). Daily inhibition of TYK2 was 91% for zasocitinib 30 mg and 23% for deucravacitinib 6 mg, and minimal TYK2 inhibition was observed for baricitinib 4 mg, upadacitinib 30 mg and tofacitinib 10 mg (0–8%; Figure 2). Simulated plasma concentrations of zasocitinib 30 mg and deucravacitinib 6 mg did not reach the JAK1/3 IC50, and those of baricitinib 4 mg, upadacitinib 30 mg and tofacitinib 10 mg remained above the JAK1/3 IC50 for 24 hours (Table 1, Figure 1). Daily inhibition of JAK1/3 was 0% for zasocitinib 30 mg and 3% for deucravacitinib 6 mg versus 91–97% for baricitinib 4 mg, upadacitinib 30 mg and tofacitinib 10 mg (Figure 2).
Conclusion: Zasocitinib exhibits selective inhibition of TYK2-mediated signaling, with greater and longer inhibition than deucravacitinib at its clinical dosage, without affecting JAK1/3-mediated signaling. These data show selective inhibition of TYK2 offers a distinct cytokine receptor inhibition profile versus when JAK is inhibited, and may contribute to an improved safety profile.
Reference:
1. Leit S et al. J Med Chem 2023;66:10473–96.
To cite this abstract in AMA style:
Mehrotra S, Sano Y, Wilson E, Durairaj C, Kong K, Xia G, Dunbar F, Spector T, Bunick C, McInnes I. Zasocitinib (TAK-279) Displays High Levels of TYK2 Inhibition and No Inhibition of JAK 1/3 Compared with Licensed TYK2 and JAK Inhibitors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/zasocitinib-tak-279-displays-high-levels-of-tyk2-inhibition-and-no-inhibition-of-jak-1-3-compared-with-licensed-tyk2-and-jak-inhibitors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zasocitinib-tak-279-displays-high-levels-of-tyk2-inhibition-and-no-inhibition-of-jak-1-3-compared-with-licensed-tyk2-and-jak-inhibitors/