Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Zasocitinib (TAK-279) is a highly selective, allosteric, oral inhibitor of tyrosine kinase 2 (TYK2). TYK2 mediates signaling from cytokines involved in the pathogenesis of psoriasis and other immune-mediated inflammatory diseases.1,2 In a recent phase 2b trial in patients with moderate-to-severe psoriasis (NCT04999839), zasocitinib was well tolerated and demonstrated safety and efficacy with greater skin clearance at doses ≥ 5 mg compared with placebo, with the two highest doses (15 mg and 30 mg) showing the strongest responses at Week 12.3 This analysis further evaluated the efficacy of the 15 mg and 30 mg doses of zasocitinib in this study using body surface area (BSA) involvement.
Methods: In this randomized, multicentre, double-blind, placebo-controlled trial, patients with moderate-to-severe plaque psoriasis were randomized 1:1:1:1:1 to receive oral zasocitinib (2 mg, 5 mg, 15 mg or 30 mg) or placebo, once daily for 12 weeks. BSA outcome measures assessed at Week 12 were mean change in BSA from baseline, mean percentage change in BSA from baseline and the proportion of patients achieving a BSA threshold of ≤ 1% by visit. Mean change in BSA from baseline was pre-specified.
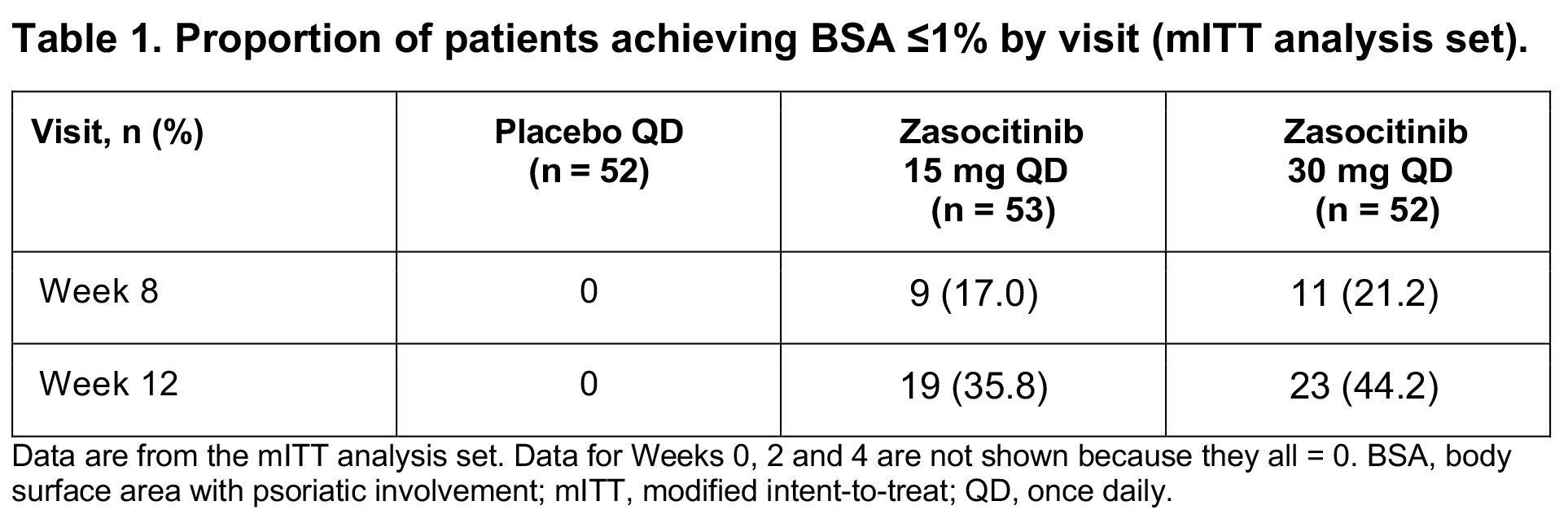
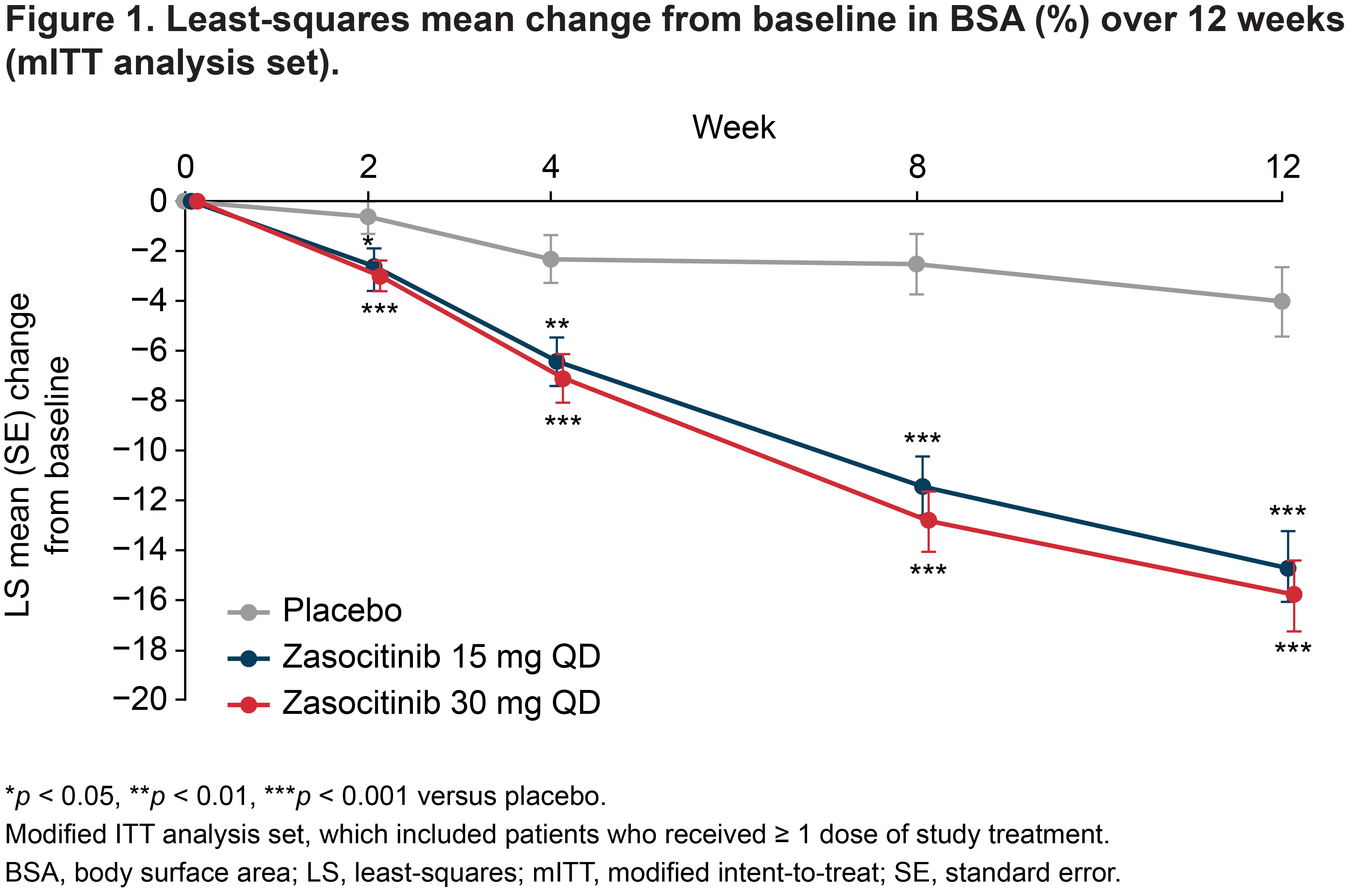
Results: Baseline mean (standard deviation [SD]) percentage BSA was generally consistent across zasocitinib 15 mg (n=53; 18.3 [10.3]), zasocitinib 30 mg (n=52; 22.2 [14.3]) and placebo (n=52; 21.3 [13.6]) groups. Mean percentage BSA decreased as early as Week 2 and continued to decrease throughout follow-up in the zasocitinib groups, whereas scores slightly decreased in the placebo group. At Week 12, mean (SD) percentage BSA was 4.4 (5.3) (least-squares [LS] mean change: −14.7; LS mean percentage change: −72.9%) in the 15 mg group, 6.5 (12.5) (LS mean change: −15.7; LS mean percentage change: −73.1%) in the 30 mg group and 18.2 (13.6) (LS mean change: −4.0; LS mean percentage change: −19.3%) in the placebo group (p < 0.001 for both doses versus placebo) (Figure 1). From Week 8 onwards, a higher proportion of patients achieved a BSA threshold of ≤ 1% in the zasocitinib 15 mg and 30 mg groups compared with the placebo group (35.8% and 44.2% versus 0% at Week 12, respectively) (Table 1).
Conclusion: Patients with moderate-to-severe plaque psoriasis who received the two highest doses of zasocitinib (15 mg and 30 mg) in the phase 2b trial achieved greater reductions in BSA than patients who received placebo over 12 weeks. Further investigation of the efficacy and safety of zasocitinib in phase 3 studies in psoriasis is ongoing (NCT0608804; NCT06108544).
References:
1. Leit S et al. J Med Chem 2023;66:10473–96.
2. Rusiñol L, Puig L. Int J Mol Sci 2023;24:3391.
3. Armstrong A et al. Oral presentation presented at the Annual Meeting of the American Academy of Dermatology, March 17–21, 2023, New Orleans, LA, USA.
To cite this abstract in AMA style:
Laquer V, Kircik L, Sadick N, Weisman J, Zhao Y, Blau J, Zhang W, Uy J, Winkelman W, Gooderham M. Zasocitinib (TAK-279), a Selective Oral Tyrosine Kinase 2 Inhibitor, Reduces Body Surface Area Involvement in a Phase 2b Trial in Moderate-to-Severe Plaque Psoriasis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/zasocitinib-tak-279-a-selective-oral-tyrosine-kinase-2-inhibitor-reduces-body-surface-area-involvement-in-a-phase-2b-trial-in-moderate-to-severe-plaque-psoriasis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/zasocitinib-tak-279-a-selective-oral-tyrosine-kinase-2-inhibitor-reduces-body-surface-area-involvement-in-a-phase-2b-trial-in-moderate-to-severe-plaque-psoriasis/