Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Several osteoporosis (OP) medicines came on the market in the past two decades, each with its own indications. However, there is no clear information on how the medicines were prescribed in the real world. Using Medicare data, we assessed who prescribed which OP medication for what type of patients.
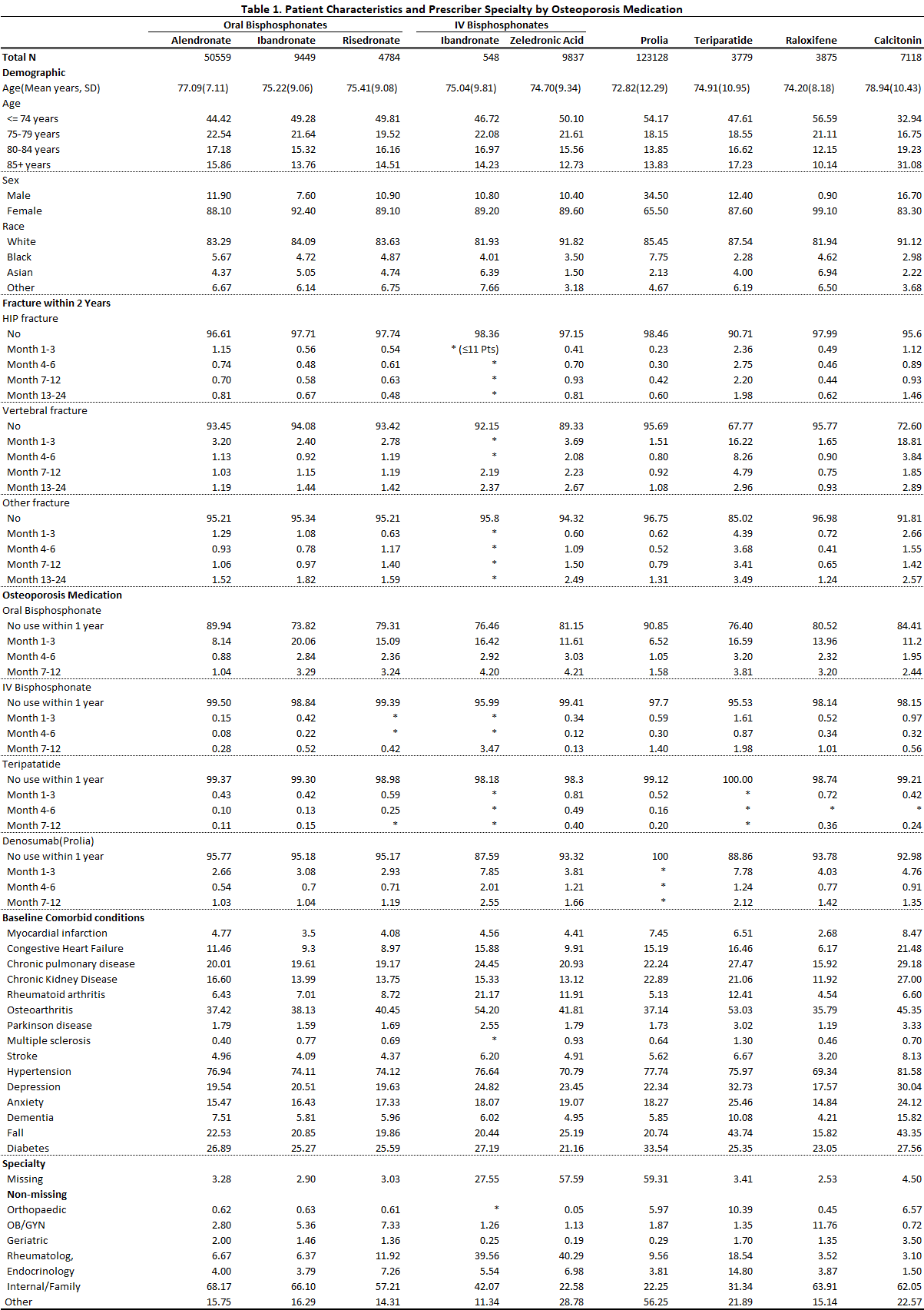
Methods: Using the 2011-2015 Medicare 20% random sample database, we identified patients who initiated an OP medication during a two years period – October 1, 2013, to September 30, 2015, and the specialty of the initial prescribers. OP medications included alendronate, ibandronate (oral and IV formats, separately), risedronate, zoledronic acid, teriparatide, denosumab, raloxifene, and calcitonin; prescriber’s specialties included orthopedic, OB/GYN, geriatric, rheumatologic, endocrinologic, internal medicine or family, and “other.” Patients were aged ≥65 years at initiation and covered by Medicare Parts A and B for at least 2 years and by Medicare Part D for at least 1 year before initiation. Initiation was defined as first use in the period and no use of the same medicine for at least 1 year before. Patients could be on other OP medications before this initiation. For each medication group, we assessed patient demographics (age, race, sex, and geographic regions), history of fractures (hip, vertebral, other), comorbid conditions, which may be associated with increased fracture risk, and previous OP medication use.
Results: In all, 203,305 patients were included; 4.6% initiated more than one medicine (one by one, not simultaneously) in this period. The largest medication group was denosumab users (123,128), followed by alendronate (50,559) users; the smallest groups were IV ibandronate (548) and teriparatide (3,779) users. Calcitonin users were the oldest (mean age 78.9 years) and denosumab users the youngest (72.8). About 10% of users were men for all medicines except for raloxifene (< 1%) and denosumab (34.5%). More calcitonin and teriparatide users were at high risk of fracture (with previous fracture or more comorbidity) and a large percentage had recent fractures, especially vertebral; more ibandronate and teriparatide users switched from (other) bisphosphonates. OP medication was mostly prescribed by internal/family (47.2%) and “other” (32.1%) doctors. However, this varied by medication; except for high percentages of internal/family and “other” doctors, about 40% of IV bisphosphonates were prescribed by rheumatologists; 10.4%, 18.5%, and 14.8% of teriparatide by orthopedists, rheumatologist, and endocrinologists; 11.8% of raloxifene by OB/GYN; and 11.9% of risedronate by rheumatologists. For details, see Table 1.
Conclusion: This study found that, in the Medicare population, denosumab was prescribed for the most patients and was used by more men; calcitonin was mainly prescribed for older women; calcitonin and teriparatide were prescribed for more patients at high risk of fracture; and OP medications were mainly prescribed by internal/family and “other” doctors. Internal medicine and family doctors should remain updated regarding osteoporosis treatment-related research results. A study limitation is that prescriber information was not complete for IV medications.
To cite this abstract in AMA style:
Liu J, Guo H, Gong T, Peng Y. Who Prescribed Which Osteoporosis Medication to Whom [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/who-prescribed-which-osteoporosis-medication-to-whom/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/who-prescribed-which-osteoporosis-medication-to-whom/