Session Information
Date: Saturday, November 6, 2021
Title: Health Services Research Poster I: Lupus, Inflammatory Arthritis, & More (0128–0148)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Annual influenza vaccination is recommended for adults with RA, but remains low in established RA. We examined influenza vaccination coverage in the year prior to and following RA diagnosis, and individual characteristics including RA medication beliefs associated with vaccination among newly diagnosed participants in the Canadian Early Arthritis Cohort (CATCH).
Methods: The sample included adults enrolled in CATCH between Dec 2017 and Mar 2021 who met 2010 ACR/EULAR criteria, had 1+ year of follow-up, and had completed the Beliefs about Medicines Questionnaire. Vaccinated and non-vaccinated groups were compared using t-tests and chi-square and multivariable logistic regression was used to identify characteristics associated with vaccination in the year prior to (pre-dx) vaccination and the first year of RA (post-dx).
Results: At enrollment, participants (N=405) were mostly white (80%) women (67%) with a mean (SD) age of 56 years and symptom duration of 5 months. Pre-dx, 37% reported being vaccinated, increasing to 42% post-dx. Among 233 patients with vaccination info available post-dx, most (68%) of those vaccinated post-dx reported having been vaccinated pre-dx.
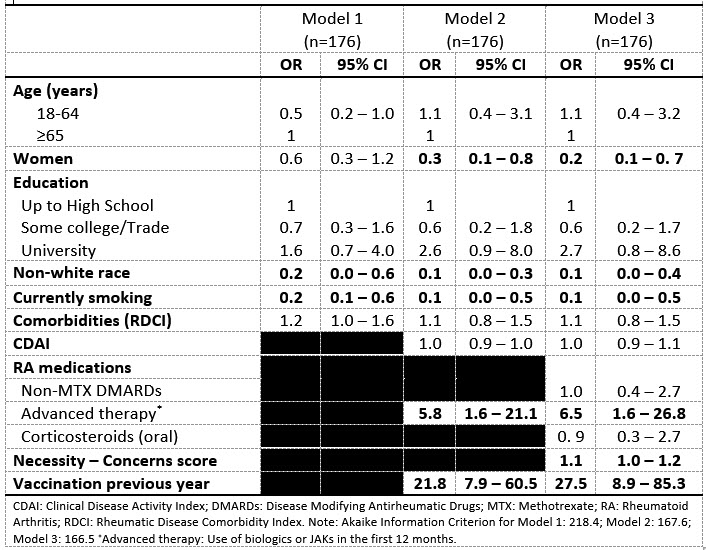
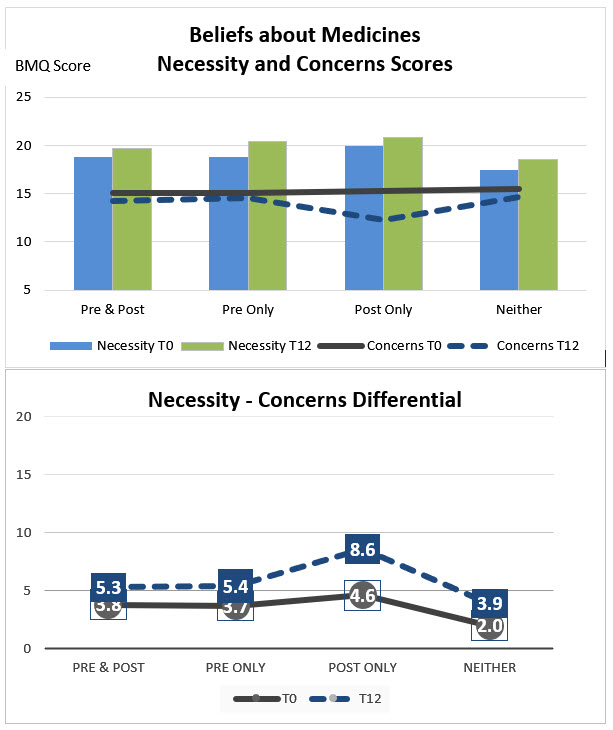
In adjusted analyses, pre-dx influenza vaccination was associated with age ≥65 and having more comorbidities, but not sex, racial background, education, smoking, CDAI, or RA medication beliefs at baseline. Post-dx vaccination was associated with male sex, white racial background, not smoking, biologics/JAK inhibitor use, higher mean Necessity-Concerns differential scores, and pre-dx vaccination (Table). Individuals who were not vaccinated pre-dx but vaccinated post-dx had lower mean Concerns and higher mean Necessity-Concerns differential scores compared to those vaccinated at both timepoints, only pre-diagnosis, or neither timepoints (Figure).
Conclusion: In Canadians with early RA, influenza vaccination coverage remains suboptimal. Certain individual (male sex, non-white race, smoking, medication beliefs/concerns) and treatment characteristics (advanced therapeutics, prior vaccination) were associated with a greater likelihood of vaccination. Higher concerns about RA medications and low necessity beliefs may also predict vaccine hesitancy. Conversations about beliefs and attitudes about RA medications and vaccination history as part of the diagnostic workup may help increase influenza vaccine coverage.
To cite this abstract in AMA style:
Ta V, Schieir O, Valois M, Colmegna I, Hitchon C, Bessette L, Hazlewood G, Thorne C, Pope J, Boire G, Tin D, Keystone E, Bykerk V, Bartlett S, Investigators C. Who Gets Influenza Vaccinations Prior to and After a Diagnosis of RA? Results from the Canadian Early Arthritis Cohort (CATCH) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/who-gets-influenza-vaccinations-prior-to-and-after-a-diagnosis-of-ra-results-from-the-canadian-early-arthritis-cohort-catch/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/who-gets-influenza-vaccinations-prior-to-and-after-a-diagnosis-of-ra-results-from-the-canadian-early-arthritis-cohort-catch/