Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Active axial spondyloarthritis (axSpA) is associated with poorer physical and mental health outcomes. This study aims to assess the prevalence of clinically active disease in axSpA and its associated factors in a large sample of patients from the International Map of Axial Spondyloarthritis (IMAS) study from around the globe.
Methods: IMAS is a cross-sectional online survey (2017-2022) including 5,557 unselected axSpA patients. Patients were divided between those with active disease (BASDAI < 4) and those without active disease (BASDAI ≥ 4). The factors evaluated were: age, gender, physical activity engagement, work-related issues, work choice and difficulty finding a job due to axSpA, number of self-reported symptomatic body regions, diagnostic delay, HLA-B27, extra-musculoskeletal manifestations, spinal stiffness (3-12), functional limitation (0-54), mental health using GHQ-12 scale (0-12), and treatments (NSAIDs, csDMARDs and bDMARDs). Mann-Whitney, chi-square test and logistic regression analysis were used to evaluate the possible association of the investigated factors with active disease in axSpA patients.
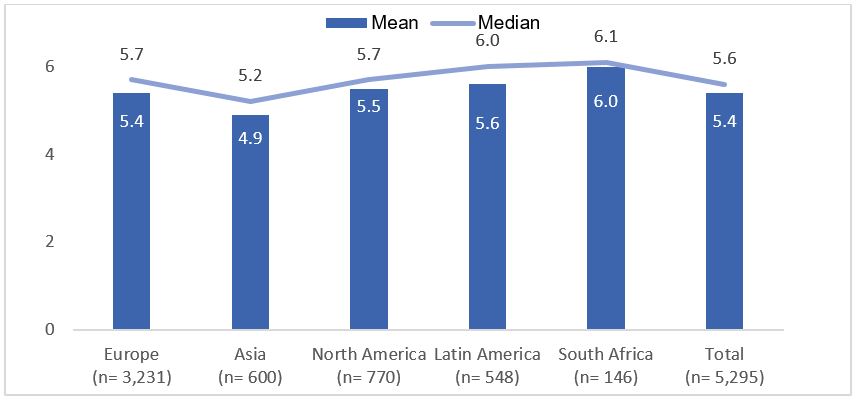
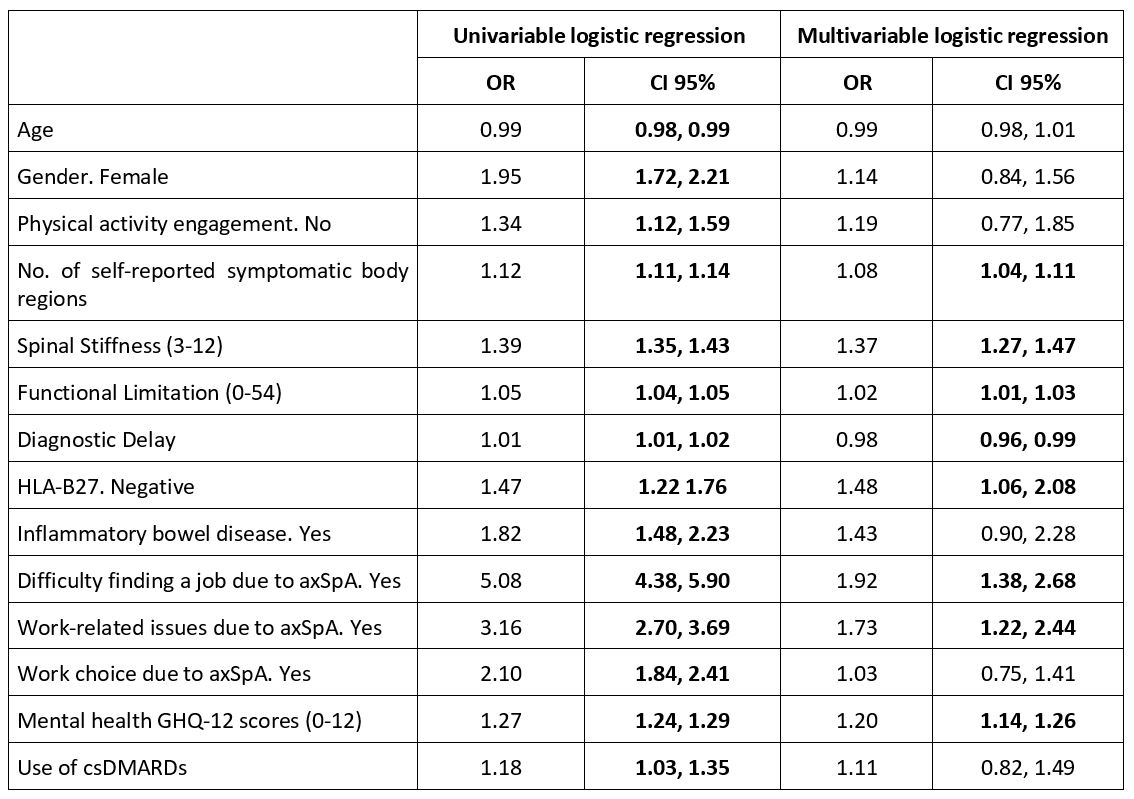
Results: 5,295 patients who had responded to the BASDAI scale in IMAS survey were included in the present study: 3,231 were from Europe, 770 from North America, 600 from Asia, 548 from Latin America, and 146 from Africa. The mean age was 43.8±12.9 years and 55.4% were females. Patient reported a mean BASDAI of 5.4 (median 5.6; Figure 1) with 75% having active disease (BASDAI≥4). In South Africa, 87.0% of patients reported having active disease, compared to 68.5% in Asia. Compared to patients with non-active disease, patients with active disease were more likely to be older, female, physically inactive, experience work-related issues, have greater difficulty finding a job and faced limited work choice due to axSpA, had higher number of self-reported symptomatic body regions, longer diagnostic delay, higher proportion of HLA-B27 negative, presence of inflammatory bowel disease, greater spinal stiffness, higher functional limitation, worse mental health, and greater use of csDMARDs. In the multivariable logistic regression, the factors associated with active disease were the presence of work-related issues due to axSpA, difficulty finding a job due to axSpA, higher number of self-reported symptomatic body regions, greater spinal stiffness, higher functional limitation, longer diagnostic delay, HLA-B27 negative, and worse mental health (Table 1).
Conclusion: Globally, three in four patients with axSpA reported clinically active disease, with higher proportion of patients with active disease in South Africa and lower in Asia. The causal relationship between the identified factors and clinical disease activity is complex and may vary from patient to patient. Our results underline the complexity of the clinical disease activity concept in axSpA and underline the need of a holistic approach in the patient management.
To cite this abstract in AMA style:
Garrido-Cumbrera M, Navarro-Compán V, Sommerfleck F, Bundy C, Makri S, Correa Fernandez J, Murlidhar Akerkar S, Davies J, Karam E, Siddiqui A, Poddubnyy D. Which Factors Are Associated with Clinically High Disease Activity in Axial Spondyloarthritis? Results from the International Map of Axial Spondyloarthritis (IMAS) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/which-factors-are-associated-with-clinically-high-disease-activity-in-axial-spondyloarthritis-results-from-the-international-map-of-axial-spondyloarthritis-imas/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-factors-are-associated-with-clinically-high-disease-activity-in-axial-spondyloarthritis-results-from-the-international-map-of-axial-spondyloarthritis-imas/