Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment III: Axial Spondyloarthritis (2028–2032)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: Several disease activity response and status criteria are used to assess treatment efficacy in RCTs in axial spondyloarthritis (axSpA). Response criteria include: the Assessment of SpondyloArthritis international Society (ASAS)-based ASAS 20, ASAS 40, and ASAS 5/6; the Bath Ankylosing Spondylitis Disease Activity Index BASDAI 50; the Ankylosing Spondylitis Disease Activity Score (ASDAS)-based clinically important improvement (ASDAS-CII) and major improvement (ASDAS-MI). Additionally, the following disease activity status are used: ASAS partial remission (ASAS-PR), ASDAS-low DA (ASDAS-LDA) and ASDAS-inactive disease (ASDAS-ID). All these 9 are variably used in RCTs, but it remains unknown which one is the most discriminative. Our aim was to compare the ability of different criteria to discriminate the response in treatment and placebo arm in axSpA.
Methods: A systematic literature review was performed in Medline and Embase to identify RCTs of biological and targeted synthetic DMARDs (b- and tsDMARDs). Placebo-controlled RCTs meeting the primary endpoint have been included provided they reported ≥2 response/status criteria. Outcomes were collected at the timepoint of primary endpoint assessment. Risk of bias was evaluated by The Cochrane tool. Meta-analysis with the Mantel-Haenszel method was conducted to calculate the Chi-square between percentages of patients fulfilling each criterion in the treatment arm versus the placebo arm (higher Chi-square, better discrimination). Per meta-analysis, we pooled RCTs presenting the same sets of outcomes.
Results: Eleven articles fulfilling inclusion criteria were retrieved from a preceding SLR about RCTs in axSpA (2001-2013), and 12 from another SLR (2009-2016) [3,4]. The search update resulted in 6 additional articles out of 130 hits. Thus, 29 RCTs in total were eligible. Meta-analysis included 23/29 RCTs with primary endpoint at 12-16 weeks, all at a low risk of bias. Other 6 RCTs had later (e.g. 24 weeks) or earlier (e.g. 6 weeks) primary endpoint, thus could not be meta-analyzed due to heterogeneity. Out of the 23 RCTs, 2 studies reported 2 outcomes, 2 reported 3 outcomes and 19 RCTs reported ≥4 outcomes. The most frequently reported outcomes were ASAS 20, ASAS 40, ASAS-PR and BASDAI 50 (Set 1). Sixteen RCTs presented all outcomes from Set 1 (Table 1): the discriminative performances were, in descending order, ASAS 40 – ASAS 20 – BASDAI 50 – ASAS-PR. In 11/16 RCTs ASAS 5/6 was additionally included (Table 1, Set 2): this outcome showed the best performances among ASAS-based response criteria. 8/16 RCTs additionally included some ASDAS-based outcomes (Table 1, Set 3). Here, ASDAS-CII and -MI showed a much higher discrimination than the ASAS-based criteria. In only 3 trials all outcomes could be compared, with again the ASDAS-CII and -MI as the most discriminative criteria, followed by ASAS 5/6 (Table 1, Set 4).
Conclusion: ASDAS-based response criteria showed better discrimination than ASAS-based response criteria. ASDAS-CII and ASDAS-MI should be the preferred primary outcomes for future RCTs comparing a bDMARD or tsDMARD with placebo, as a smaller sample size can be used with the same statistical power, in comparison to the currently most frequently used ASAS 40.
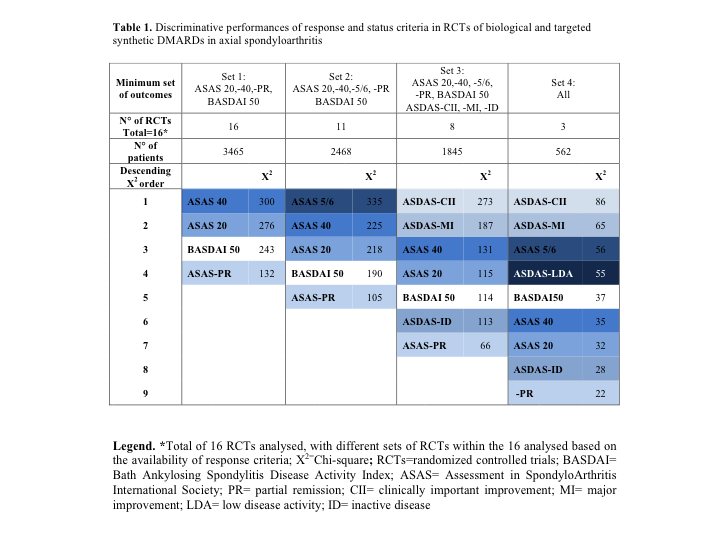 Discriminative performances of response and status criteria in RCTs of biological and targeted synthetic DMARDs in axial spondyloarthritis
Discriminative performances of response and status criteria in RCTs of biological and targeted synthetic DMARDs in axial spondyloarthritis
To cite this abstract in AMA style:
Ortolan A, Navarro-Compán V, Sepriano A, Landewé R, van der Heijde D, Ramiro S. Which Disease Activity Outcome Measure Discriminates Best in Axial Spondyloarthritis? A Systematic Literature Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/which-disease-activity-outcome-measure-discriminates-best-in-axial-spondyloarthritis-a-systematic-literature-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/which-disease-activity-outcome-measure-discriminates-best-in-axial-spondyloarthritis-a-systematic-literature-review-and-meta-analysis/
