Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Tumour necrosis factor inhibitors (TNFi) and Interleukin-17Ai inhibitors (IL-17Ai) have shown effectiveness in achieving low disease activity (LDA) in patients with axial spondyloarthritis (axSpA). The impact of prior TNFi treatment on the time it takes to achieve LDA and the duration of achieved LDA in subsequent TNFi or IL-17Ai treatment remains unknown. Thus, we aimed to assess the time to achieve LDA from the initiation of a TNFi or an IL-17Ai, as well as the duration of achieved LDA, in patients with axSpA and 0, 1 or 2 prior TNFi treatments.
Methods: Patients initiating an IL-17Ai or a TNFi between 2017 and 2023 were included from 13 registries in the EuroSpA Research Collaboration. We excluded patients with LDA at treatment start or prior treatment with targeted synthetic disease-modifying anti-rheumatic drugs (tsDMARDs) or non-TNFi/non-IL-17Ai biological DMARDs.
LDA was defined as the Axial Spondyloarthritis Disease Activity score (ASDAS) < 2.1. Time to achieve LDA was defined as the number of weeks from treatment start to the first visit in LDA, and durability as the number of weeks between the first visit in LDA and the first subsequent visit not in LDA. Patients not achieving LDA during follow-up were censored at the last visit on treatment.
Missing components of ASDAS were imputed by predictive mean matching, and analyses were performed in separate cohorts of patients initiating TNFi and IL-17Ai, stratified by prior TNFi treatment (0/1/2). Mean (SD) and Cox regression models were applied to assess the achievement of LDA and the maintenance of LDA and compare across strata.
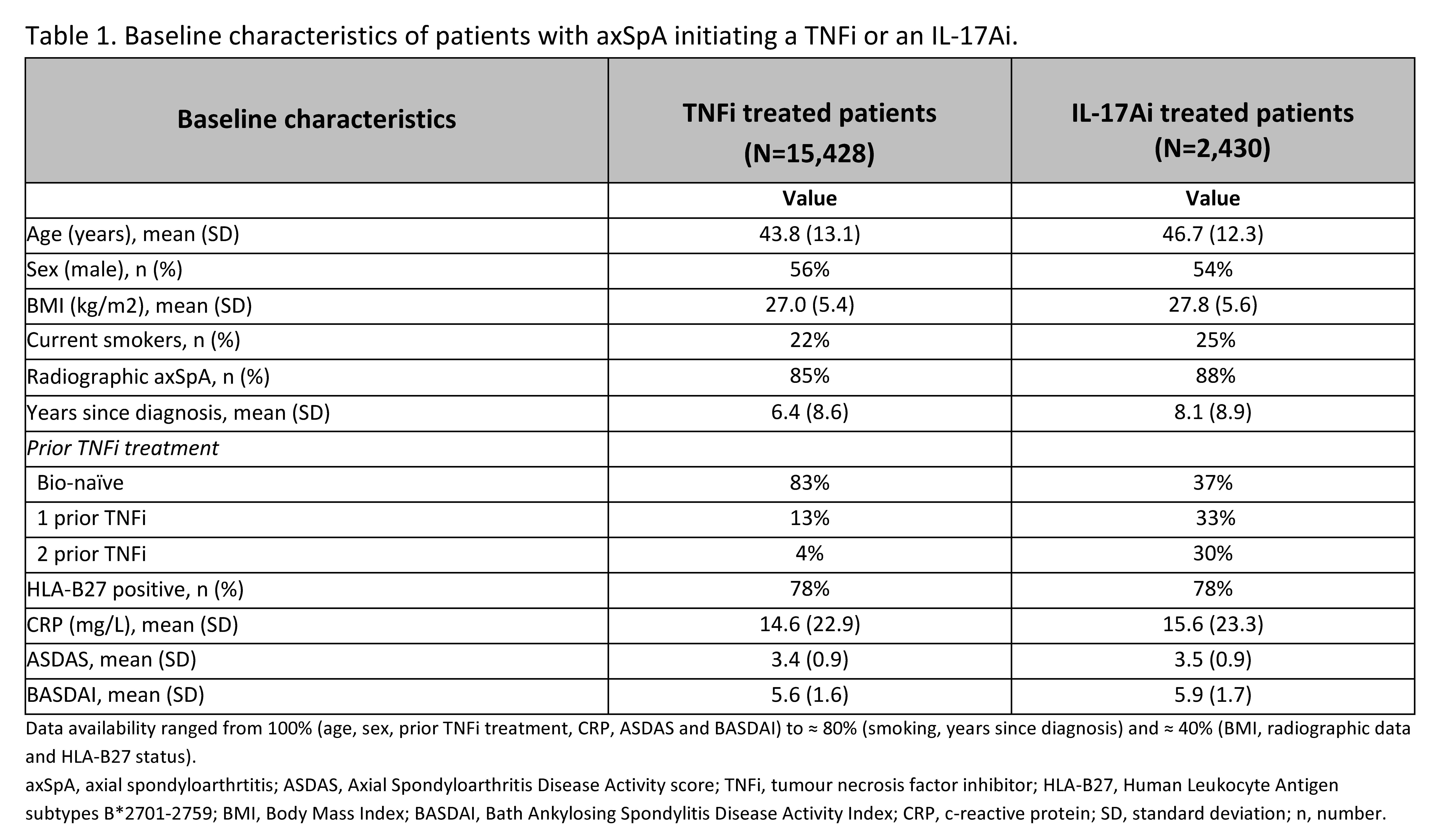
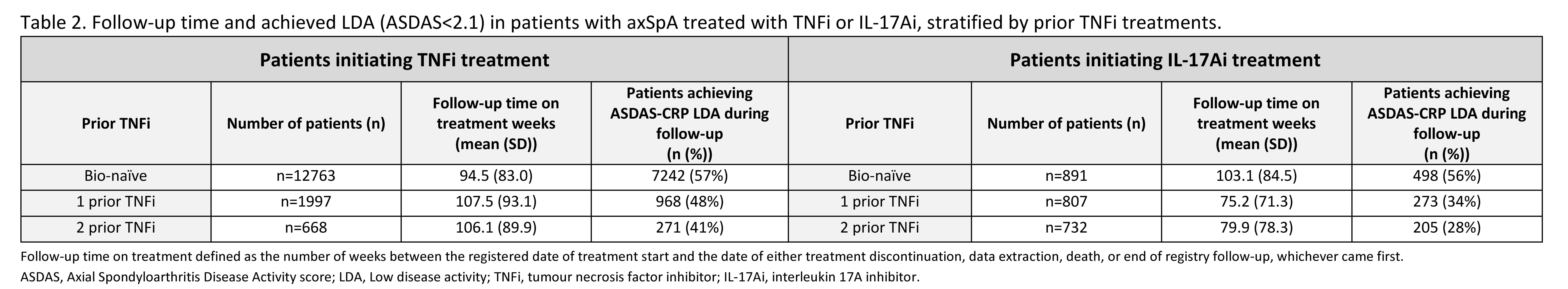
Results: We included 15,428 and 2,430 patients initiating TNFi and IL-17Ai, respectively. Baseline characteristics were largely similar apart from a higher proportion of bio-naïve patients (83% vs. 37%) in the TNFi cohort (Table 1). In both cohorts, bio-naïve patients more often achieved LDA than TNFi-experienced (Table 2).
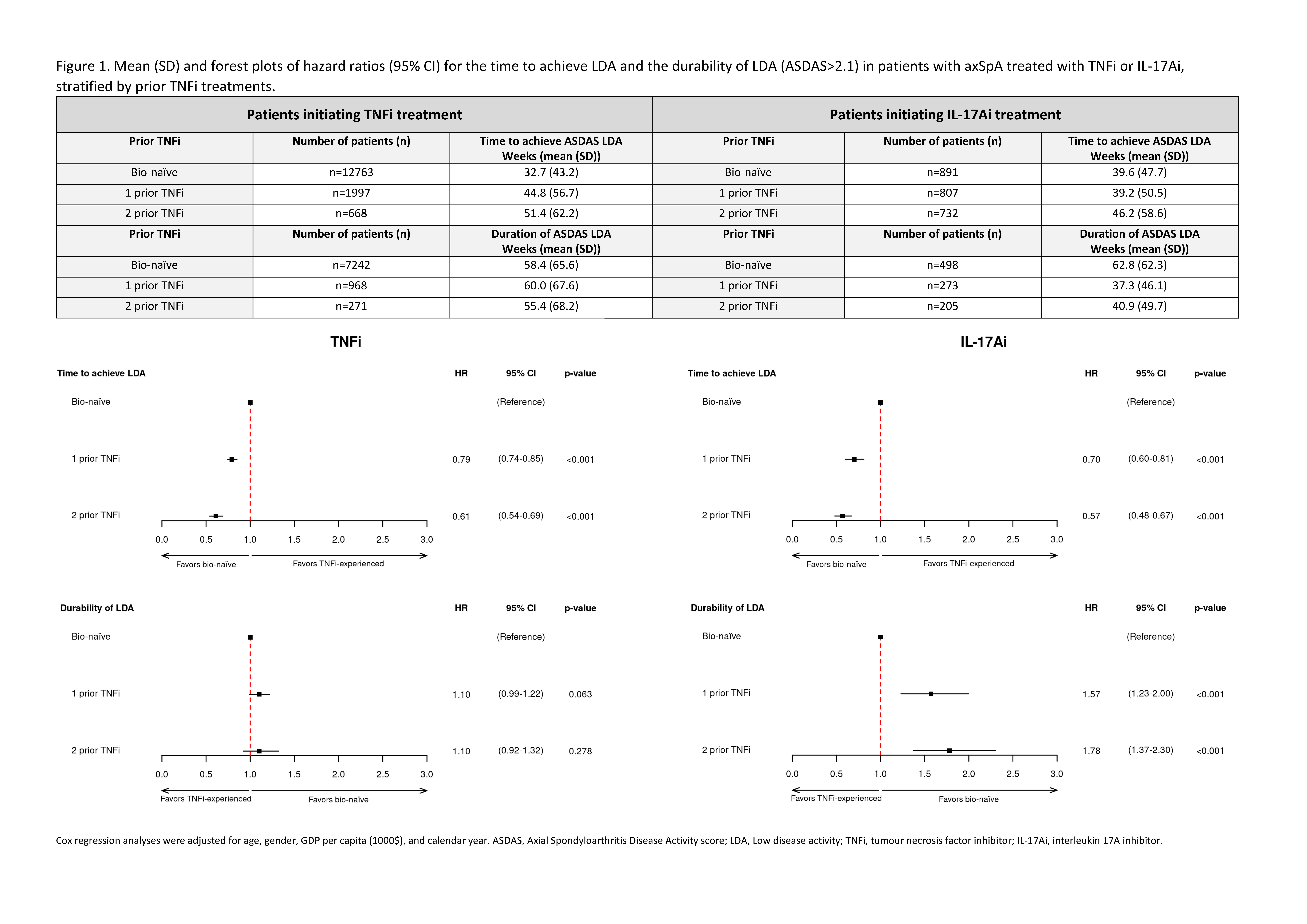
The mean time to achieve LDA across prior TNFi treatment (0/1/2) was 32.7/44.8/51.4 weeks for the TNFi cohort and 39.6/39.2/46.2 weeks for the IL-17Ai cohort (Fig. 1). With bio-naïve as reference, hazard ratios (HRs) for achieving LDA with 1 and 2 prior TNFi were, respectively, 0.79 (95% CI 0.74-0.85) and 0.61 (95% CI 0.54-0.69) in the TNFi cohort, and 0.74 (95% CI 0.63-0.86) and 0.58 (95% CI 0.48-0.69) in the IL-17Ai cohort (Fig. 1). The mean duration of LDA across prior TNFi treatment (0/1/2) was 58.4/60.0/55.4 weeks for the TNFi cohort and 62.8/37.3/40.9 weeks for the IL-17Ai cohort (Fig. 1). With bio-naïve as reference, HRs for not maintaining LDA were, respectively, 1.10 (95% CI 0.99-1.22) and 1.10 (95% CI 0.92-1.32) for 1 and 2 prior TNFi in the TNFi cohort, and, respectively, 1.57 (95% CI 1.23-2.00) and 1.78 (95% CI 1.37-2.30) for 1 and 2 prior TNFi in the IL-17Ai cohort (Fig. 1)).
Conclusion: This large European real-world cohort of patients with axSpA demonstrated that the time to achieve LDA increased with the number of prior TNFi in both TNFi and IL-17Ai treatments. While the duration of LDA was shorter for TNFi-experienced than bio-naïve patients initiating IL-17Ai, prior TNFi did not affect durability in patients initiating TNFi. More research is needed to investigate the underlying reasons for these findings.
To cite this abstract in AMA style:
Heberg J, Georgiadis S, Pons M, Loft A, Michelsen B, Linde L, DiGuiseppe D, Rasmussen S, Kazemi M, Macfarlane G, Jones G, Laas K, Vorobjov S, Castrejon I, Rotar Z, Perdan-Pikmajer K, Šenolt L, Baranová J, Glintborg B, Ciurea A, Bernardes M, Valente P, Gudbjornsson B, Gröndal G, Bakland G, Codreanu C, Mogosan C, Iannone F, Caporali R, Karlsson Wallman J, Rantalaiho V, Peltomaa R, Pavelka K, Horak P, Esperança Almeida D, Rodrigues S, Oernbjerg L, Ostergaard M, Hetland M. What Is the Impact of Prior TNF Inhibitor Treatment on the Time to Achieve Low Disease Activity and the Durability of Low Disease Activity? Real-world Results Based on 17 858 European Patients with Axial Spondyloarthritis Initiating a TNF Inhibitor or an IL-17A Inhibitor [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/what-is-the-impact-of-prior-tnf-inhibitor-treatment-on-the-time-to-achieve-low-disease-activity-and-the-durability-of-low-disease-activity-real-world-results-based-on-17-858-european-patients-with-ax/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-is-the-impact-of-prior-tnf-inhibitor-treatment-on-the-time-to-achieve-low-disease-activity-and-the-durability-of-low-disease-activity-real-world-results-based-on-17-858-european-patients-with-ax/