Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Initiating treatment in patients with psoriasis (PsO) who have unrecognized Psoriatic arthritis (PsA) may slow disease progression and improve long-term outcomes. Several candidate biomarkers are currently being studied to identify patients with unrecognized PsA. A biomarker test will ultimately only be successful if it is used – not just because of its clinical utility, but also its value which leads to reimbursement by payors. With product development process for biomarkers being expensive and subject to uncertainties, Early Health Technology Assessment (eHTA) can help indicate the commercial potential of a product to guide decisions in development decision-making. This study aimed to identify the required performance metrics that potential PsA biomarkers would need to achieve in order to be considered cost-effective.
Methods: We developed further an existing Markov model informed by literature and expert opinion. The model followed a cohort of patients aged 45-years with moderate PsO seen at a dermatology clinic and in which PsA was prevalent but unrecognized. In the biomarker arm, candidate biomarker tests were assumed to be administered at baseline, and patients who screened positive would accept combination conventional Disease-Modifying Antirheumatic Drug (cDMARD) and biologic treatment to slow disease progression. In the current practice are, patients with PsA were assumed to be clinically detected. Disease progression was modeled as linear changes in Health Assessment Questionnaire (HAQ) and PASI scores. We assumed a range of values for sensitivity, specificity, and biomarker price based on currently development progress. The time horizon was 40 years. Scenario analyses considered biomarker use in patients with mild and severe PsO separately.
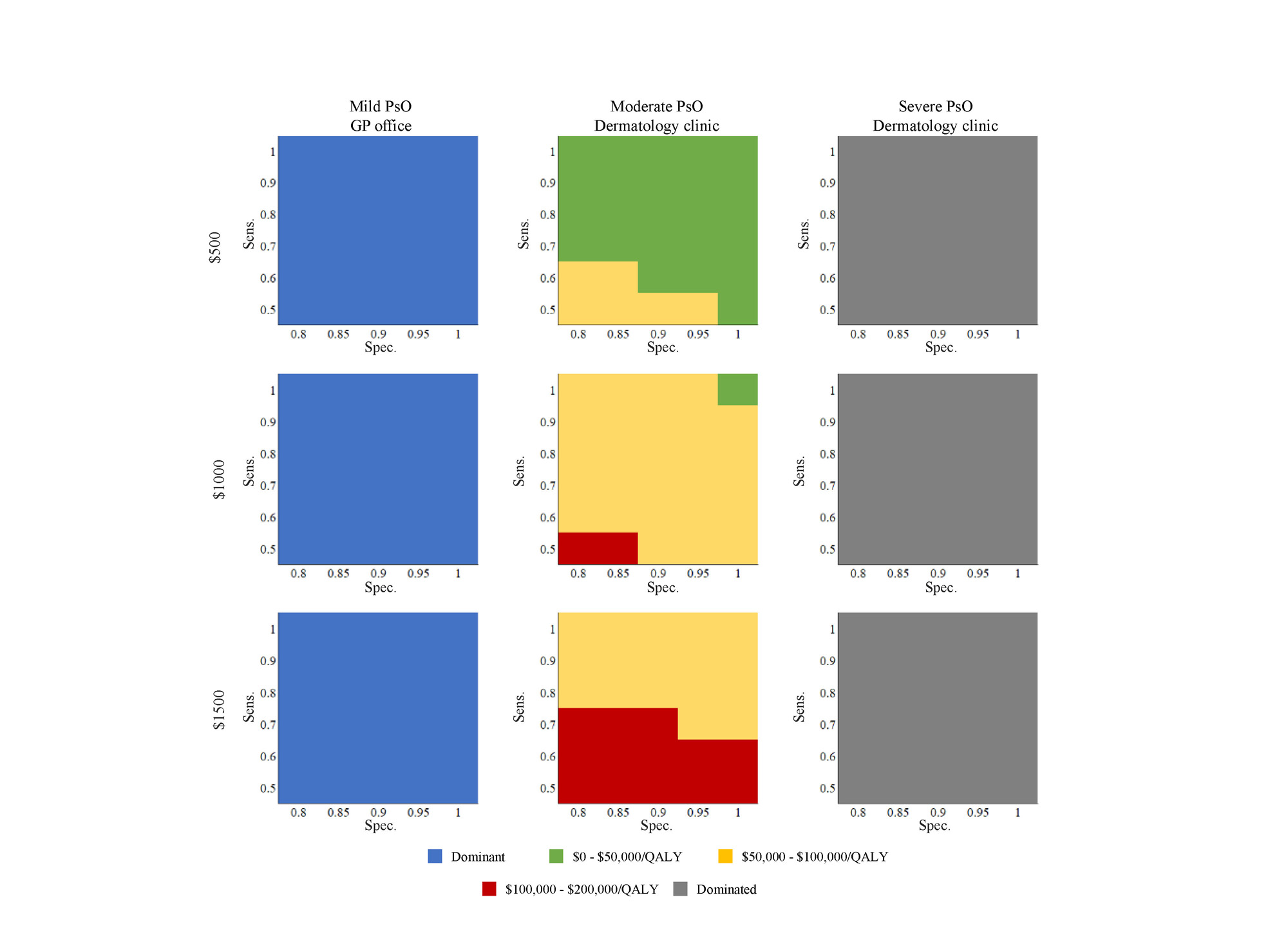
Results: In the base case, using a biomarker test with a sensitivity of 70%, specificity of 80% and a price of $500 was associated with increased cost of $817.49 and additional 0.02 QALY per patient compared to no screening (ICER $47,566.29). Multi-way analyses showed that sensitivity could be as low as 60% and considered cost-effective if specificity was at least 88% and the biomarker was priced at $500. At a price of $1,000, only a near perfect test would be considered cost-effective. Model results were sensitive to the HAQ progression under combination treatment, cDMARD costs, and the assumed start time of biologic DMARD treatment. Using a biomarker test in patients with mild PsO seen in primary care was a dominant strategy while screening in patients with severe PsO seen at a dermatology clinic was a dominated strategy (more benefit at less cost) compared to no screening.
Conclusion: This eHTA found that future PsA biomarker tests can be considered cost-effective if they can achieve modest performance, are used in a patient population with moderate PsO, and priced appropriately. Results support the continued product development of biomarker tests, and provide thresholds to guide decision-making on which biomarkers to pursue.
To cite this abstract in AMA style:
Bansback N, Tam A, Gladman D, Kulasingam V, Spackman E, Chandran V. What Are the Characteristics of a Cost-Effective Psoriatic Arthritis Biomarker Test? An Early Health Technology Assessment [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/what-are-the-characteristics-of-a-cost-effective-psoriatic-arthritis-biomarker-test-an-early-health-technology-assessment/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/what-are-the-characteristics-of-a-cost-effective-psoriatic-arthritis-biomarker-test-an-early-health-technology-assessment/