Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Pooled data from the Phase 2 AURA-LV and Phase 3 AURORA 1 studies demonstrated that adding voclosporin, a novel calcineurin inhibitor, to mycophenolate mofetil (MMF) and low-dose steroids resulted in significantly higher complete renal response rates at 24 weeks in AURA-LV (32.6% vs 19.3%; odds ratio [OR] 2.03; p=0.045) and 52 weeks in AURORA 1(40.8% vs 22.5%; OR 2.65; p< 0.0001) in patients with lupus nephritis.
The European League Against Rheumatism and European Renal Association (EULAR/ERA) published updated treatment recommendations for lupus nephritis with targeted reductions in proteinuria over the course of the first year of therapeutic intervention. Here we report on a post-hoc analysis of pooled data from the similarly-designed 48-week AURA-LV and 52-week AURORA 1 studies based on the recommended treatment targets.
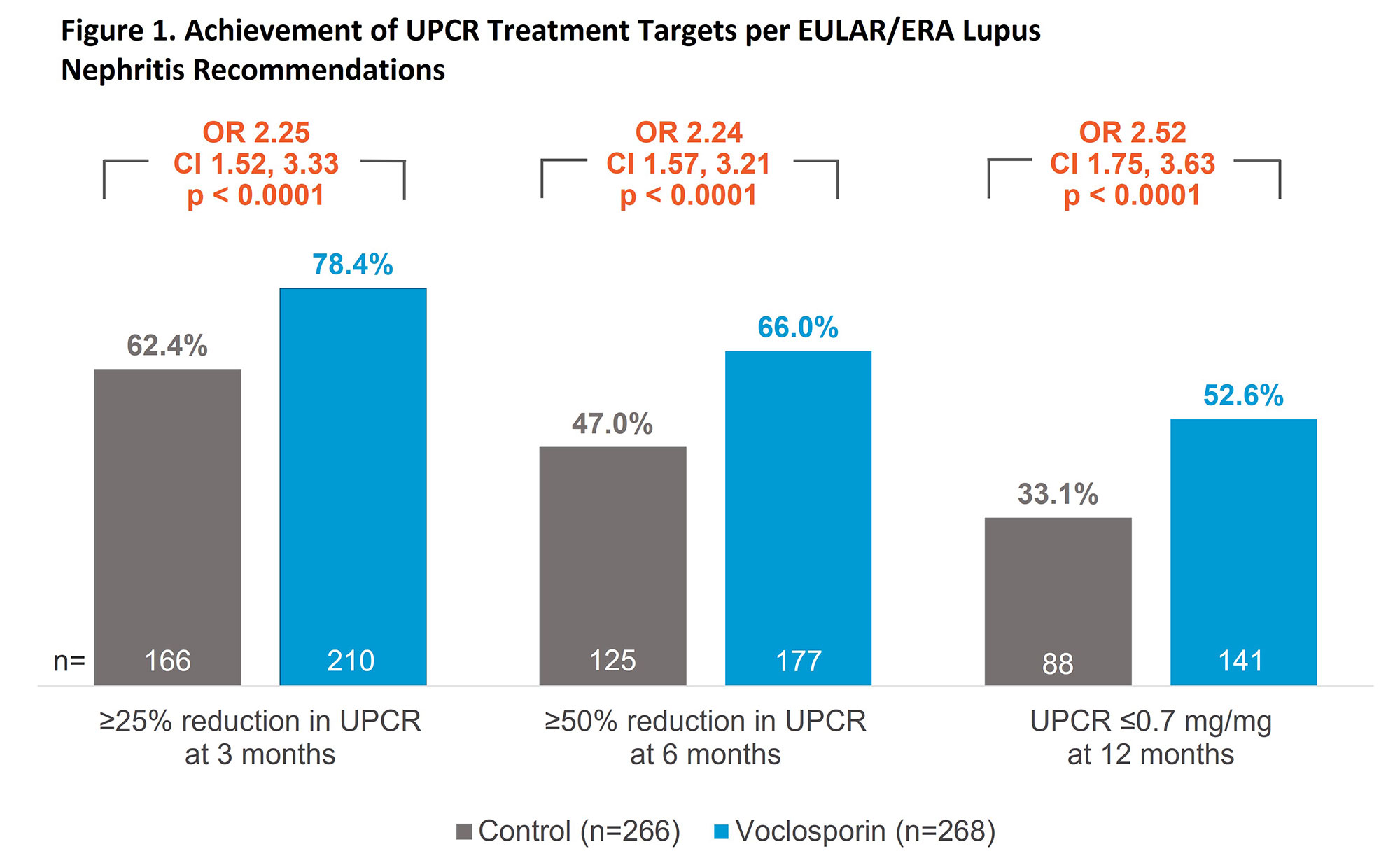
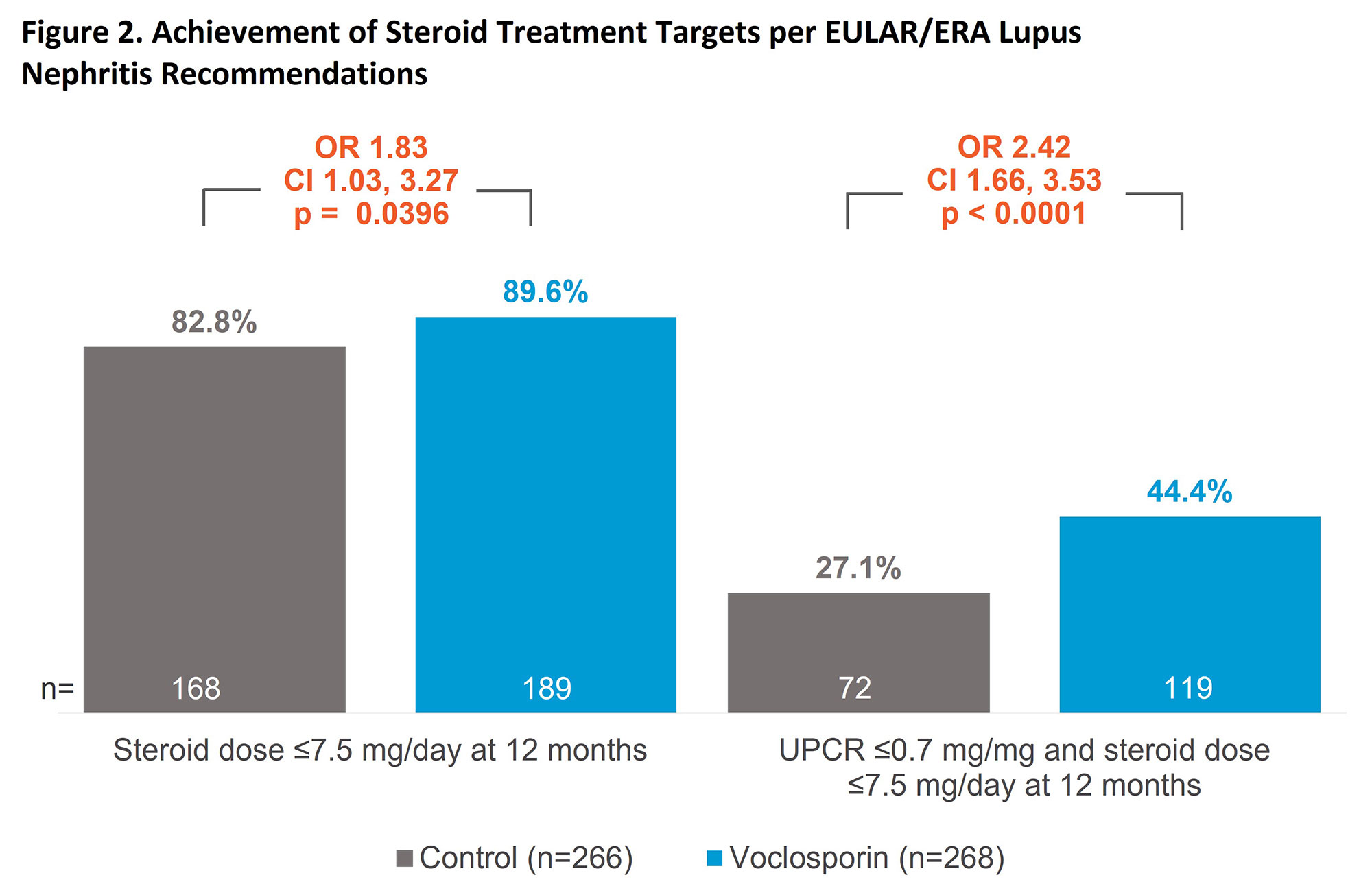
Methods: AURA-LV and AURORA 1 enrolled patients with biopsy-proven active lupus nephritis (Class III, IV, or V ± III/IV) and proteinuria ≥1.5 mg/mg (≥2 mg/mg for Class V). Pooled data included 268 patients in the voclosporin arm and 266 patients in the control arm; all patients received MMF (target dose 2 g/day) and low-dose steroids (target dose 2.5 mg/day by week 16 according to protocol-defined steroid taper). We assessed the following EULAR/ERA treatment targets: ≥25% reduction in urine protein creatinine ratio (UPCR) at 3 months, ≥50% reduction in UPCR at 6 months, UPCR ≤0.7 mg/mg at 12 months, and steroid dose ≤7.5 mg/day at 12 months.
Results: After;3 months of treatment, 78.4% of patients in the voclosporin group and 62.4% of patients in the control group achieved ≥25% reduction in UPCR (odds ratio [OR] 2.25; 95% confidence interval [CI] 1.52, 3.33; p< 0.0001). The percentage of patients achieving a reduction of ≥50% in UPCR at 6 months was also significantly greater in the voclosporin arm (66.0% vs 47.0%, respectively; OR 2.24; CI 1.57, 3.21; p< 0.0001). At 12 months, 52.6% and 33.1% of the voclosporin and control arms, respectively, had achieved a UPCR ≤0.7 mg/mg (OR 2.52; CI 1.75, 3.63; p< 0.0001). A total of 89.6% and 82.8% of patients in the voclosporin and control arms, respectively, had reached the recommended steroid dose of ≤7.5 mg/day at 12 months. The proportion of patients achieving a UPCR ≤0.7 mg/mg and having a steroid dose ≤7.5 mg/day at 12 months was 44.4% in the voclosporin arm and 27.1% in the control arm (OR 2.42; CI 1.66, 3.53; p< 0.0001).
Conclusion: The addition of voclosporin to a background regimen of MMF and low-dose steroids in patients with lupus nephritis significantly increased the likelihood of achieving the 3-, 6-, and 12-month UPCR targets of therapy recommended by EULAR/ERA.
To cite this abstract in AMA style:
Anders H, Federico R, Birardi V, Leher H. Voclosporin Is Effective in Achieving Proteinuria Treatment Targets in Lupus Nephritis Defined by EULAR/ERA Recommendations [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/voclosporin-is-effective-in-achieving-proteinuria-treatment-targets-in-lupus-nephritis-defined-by-eular-era-recommendations/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/voclosporin-is-effective-in-achieving-proteinuria-treatment-targets-in-lupus-nephritis-defined-by-eular-era-recommendations/