Session Information
Date: Monday, November 8, 2021
Title: Plenary III (1424–1429)
Session Type: Plenary Session
Session Time: 10:45AM-11:00AM
Background/Purpose: Voclosporin, a novel calcineurin inhibitor (CNI), has been tested successfully in 2 pivotal trials in adult patients with lupus nephritis (LN). Previously reported results from the Phase 3 AURORA 1 and Phase 2 AURA-LV studies showed that compared with mycophenolate mofetil (MMF) and low-dose steroids alone, the addition of voclosporin significantly increased the renal response rate and reduced proteinuria, as measured by urine protein creatinine ratio (UPCR), in patients with LN at 48 weeks in AURA-LV and 52 weeks in AURORA 1.
Methods: Here we report on the second interim analysis of the ongoing 2-year, blinded, controlled extension study, AURORA 2, with exposure up to 30 months (12 months from AURORA 1 and up to an additional 18 months in AURORA 2). All patients enrolled in AURORA 1 had a diagnosis of systemic lupus erythematosus according to the ACR criteria and biopsy-proven LN; patients completing AURORA 1 were eligible to continue in AURORA 2 with the same randomized treatment of voclosporin (23.7 mg BID) or placebo, in combination with MMF (1 g BID) and low-dose oral steroids. UPCR and estimated glomerular filtration rate (eGFR) were evaluated. In total, 116 patients in the voclosporin arm and 100 patients in the control arm enrolled in the extension study; 90 and 78 patients, respectively, had received 30 months of total treatment as of this interim analysis.
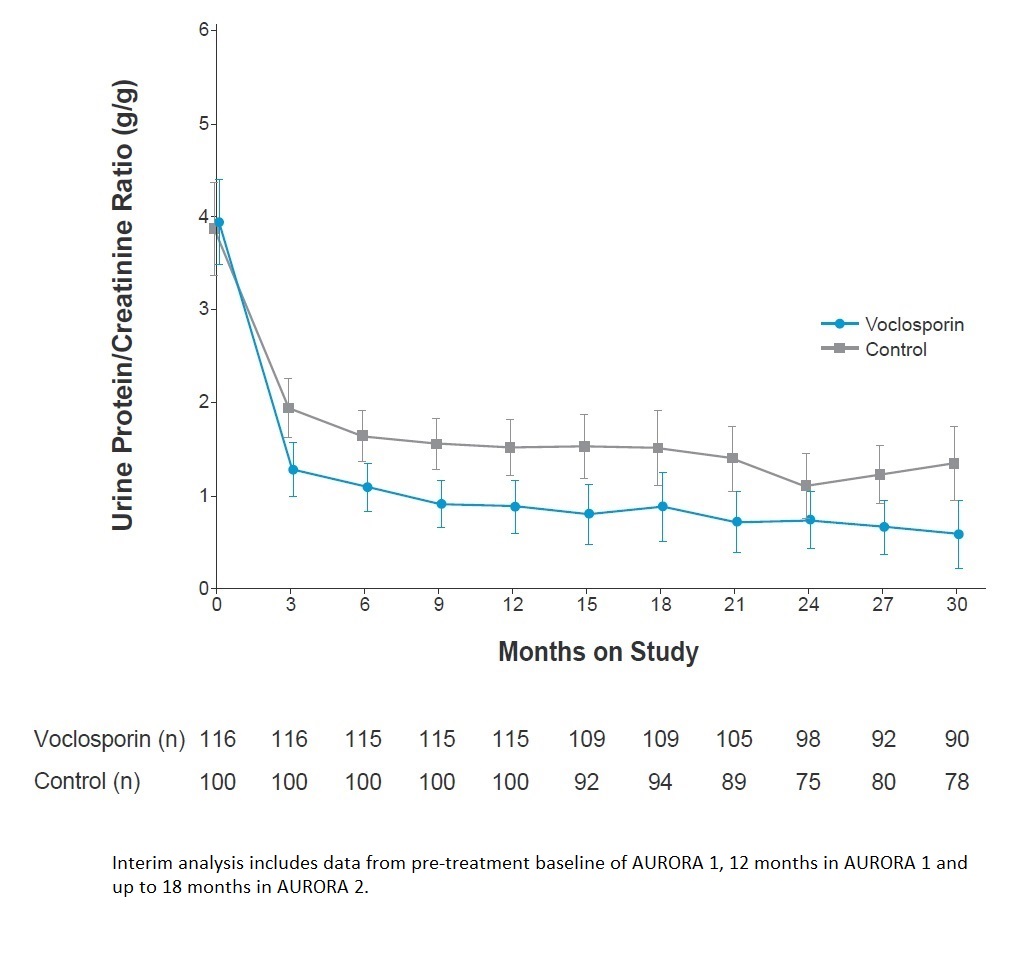
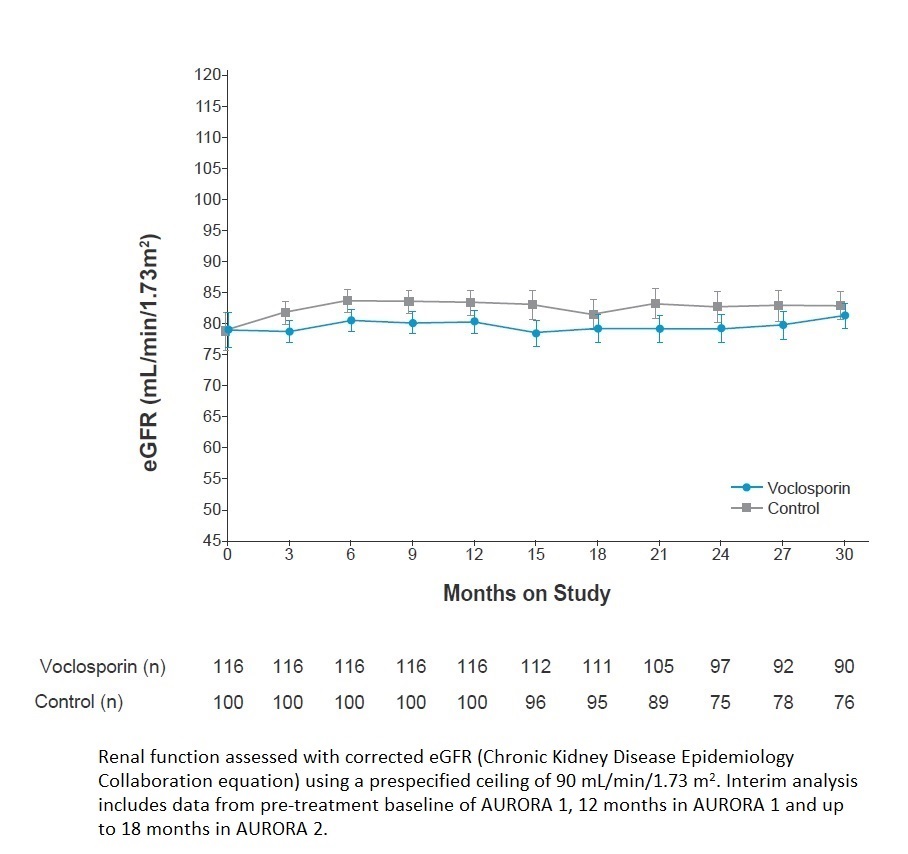
Results: Mean UPCR at pre-treatment (AURORA 1) baseline was 3.94 mg/mg in the voclosporin arm (n=116) and 3.87 mg/mg in the control arm (n=100). The LS mean change in UPCR from pre-treatment baseline to month 30 was -3.32 mg/mg for the voclosporin arm (n=90) and -2.55 mg/mg for the control arm (n=78; Figure 1). There was a small, expected and early decrease in mean eGFR in the voclosporin arm in the first 4 weeks of treatment in AURORA 1, after which eGFR remained stable through month 30 (Figure 2). There were no unexpected new adverse events reported in patients who continued voclosporin treatment compared to control-treated patients. A total of 6 and 10 patients in the voclosporin and control arms reported events of coronavirus (COVID-19) infection, with 2 and 6 patients, respectively, reporting serious coronavirus infections.
Conclusion: Patients in the voclosporin treatment arm maintained meaningful reductions in proteinuria with no change in mean eGFR at 30 months of treatment. Additional AURORA 2 efficacy and safety data will be provided at the conclusion of the study.
To cite this abstract in AMA style:
Saxena A, Mela C, Coeshall A. Voclosporin for Lupus Nephritis: Interim Analysis of the AURORA 2 Extension Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/voclosporin-for-lupus-nephritis-interim-analysis-of-the-aurora-2-extension-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/voclosporin-for-lupus-nephritis-interim-analysis-of-the-aurora-2-extension-study/