Session Information
Date: Sunday, November 7, 2021
Title: Plenary II (0956–0961)
Session Type: Plenary Session
Session Time: 10:45AM-11:00AM
Background/Purpose: In observational studies, vitamin D has been inconsistently associated with reduced risk of several autoimmune diseases, and a large randomized, controlled trial has been lacking. Dietary marine-derived long-chain omega-3 (n−3) fatty acids decrease systemic inflammation and ameliorate symptoms in some autoimmune diseases, but no trials have tested whether supplementation lowers risk of developing autoimmune disease. We tested both vitamin D3 and n-3 fatty acids for the prevention of autoimmune disease within a large nationwide randomized, controlled trial.
Methods: VITamin D and OmegA-3 TriaL (VITAL), a U.S. nationwide randomized, double-blind, placebo-controlled trial, enrolled men at least 50 years and women at least 55 years of age in a two-by-two factorial design. Randomization to vitamin D3 (2000 IU/d) and/or n-3 fatty acids (1000 mg/d) or placebo occurred from November 2011 to March 2014, and treatment continued through December 2017. (VITAL parent trial for cancer and cardiovascular disease prevention was reported in NEJM, January 3, 2019). We tested effects of vitamin D3 and n-3 fatty acids upon autoimmune disease incidence. Incident doctor-diagnosed autoimmune diseases were reported by participants annually and confirmed by medical record review by expert physicians for classification criteria (if existing). The primary endpoint was total incident autoimmune diseases, including rheumatoid arthritis, polymyalgia rheumatica, autoimmune thyroid disease, psoriasis, and all others. Pre-specified secondary endpoints included individual most common autoimmune diseases; and probable autoimmune disease (evidence of incident autoimmune disease but lacking enough medical record data to confirm). Results were displayed in cumulative incidence curves and Cox regression models calculated hazard ratios (HR) of incident autoimmune diseases.
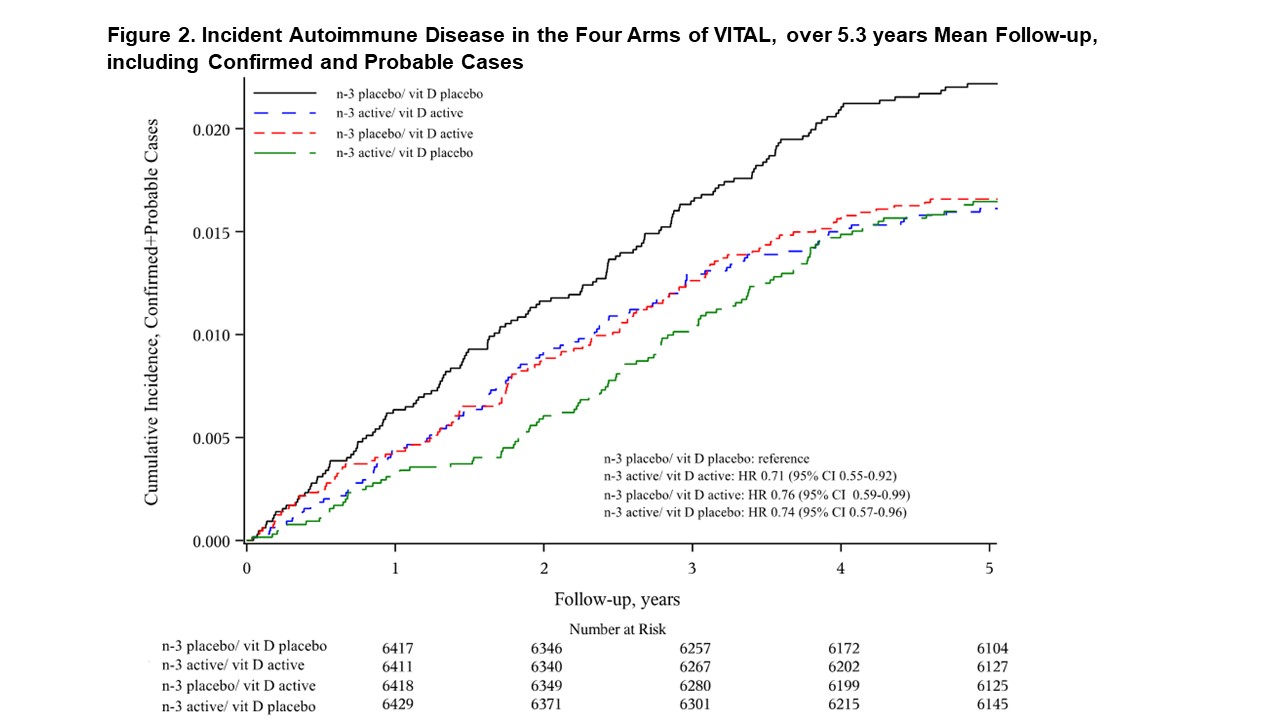
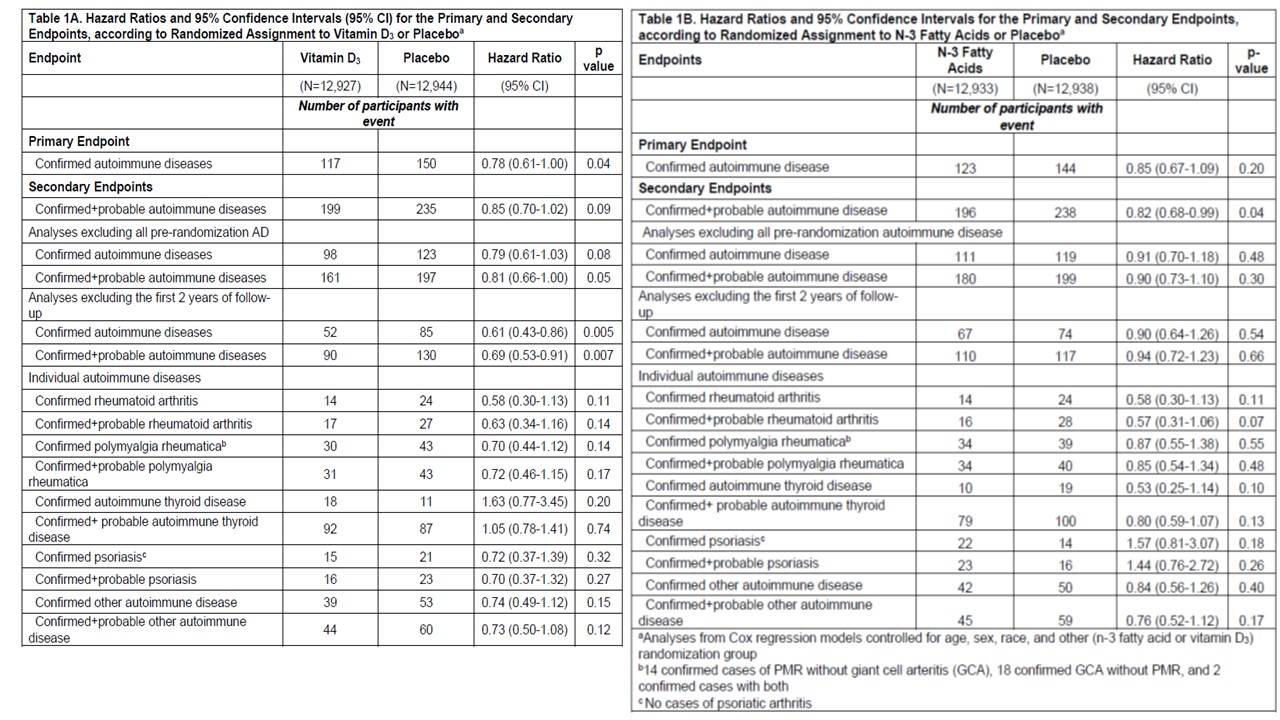
Results: 25,871 participants were randomized: 71% self-reported non-Hispanic Whites, 20% Black, and 9% other racial/ethnic groups, 51% women, mean age 67.1 years. During median follow-up of 5.3 years, confirmed autoimmune disease was diagnosed in 117 participants in the vitamin D3 group and 150 in the placebo group (HR 0.78, 95% confidence interval 0.61-1.00, p=0.04). Excluding the first 2 years in pre-specified analyses of the primary endpoint, the HR for vitamin D3 was 0.61 (0.43 – 0.86; 137 cases). Confirmed autoimmune disease was diagnosed in 123 participants in the n-3 fatty acids group and 144 in the placebo group (HR 0.85 (0.67-1.09). Excluding the first 2 years, the HR for the primary endpoint was 0.90 (0.64-1.26). (Table 1) When analyzed by factorial design subgroups, HRs for all three active arms vs. placebo/placebo were reduced by 25-30% (Figures 1-2). The number needed to treat with both agents for 5 years to prevent one autoimmune disease was 167 (94-769).
Conclusion: Supplementation for 5 years with vitamin D3 and/or n-3 fatty acids reduced incident autoimmune disease by 25-30% in older adults vs. those who received neither supplement. The effect of vitamin D3 appeared stronger after 2 years of supplementation.
To cite this abstract in AMA style:
Hahn J, Cook N, Alexander E, Friedman S, Bubes V, Walter J, Kotler G, Lee I, Manson J, Costenbader K. Vitamin D and Marine n-3 Fatty Acid Supplementation and Prevention of Autoimmune Disease in the VITAL Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/vitamin-d-and-marine-n-3-fatty-acid-supplementation-and-prevention-of-autoimmune-disease-in-the-vital-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vitamin-d-and-marine-n-3-fatty-acid-supplementation-and-prevention-of-autoimmune-disease-in-the-vital-randomized-controlled-trial/