Session Information
Date: Saturday, November 6, 2021
Title: Fibromyalgia & Other Clinical Pain Syndromes Poster (0118–0127)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Safe and effective therapies for fibromyalgia (FM) remain a major unmet clinical need. Vestibulocortical stimulation (VCS) via caloric irrigation is a safe, inexpensive, non-invasive and non-pharmacologic method of brain stimulation with demonstrated analgesic effects in persistent pain syndromes. VCS has also improved mood in psychiatric & healthy participants. Here we investigated whether VCS can similarly reduce pain & improve subjective well-being in FM patients.
Methods: We conducted a convenience-based non-randomized open-label pilot trial in 16 FM patients (mean age = 47.1 years; 15 female) recruited from a single rheumatology department. All patients met criteria for FM — defined as ≥7 widespread pain index (WPI) with ≥5 symptom severity score (SSS) or ≥4 WPI with ≥9 SSS. Half of the patient sample had primary FM, while underlying inflammatory disorders were present in 50%. Each participant underwent VCS with 50 cc of cold-water irrigation (4°C) into the right ear at 1–2 cc/second (which induces predominantly left-hemisphere activation). Vestibular stimulation was confirmed by post-procedural nystagmus & subjective vertigo. Pain scores (0–10) were recorded at baseline (before VCS) and at 5min, 15min & 30min after VCS; overall subjective well-being (0–10) was also assessed at baseline & 30min post-procedure. The primary outcome measure was change in mean pain scores collected each day/week following the VCS procedure. There were several secondary outcome measures, including immediate change in pain & subjective well-being after VCS.
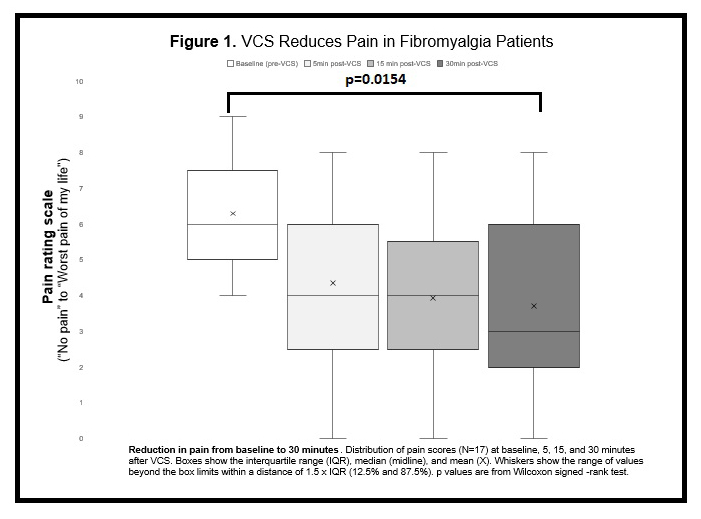
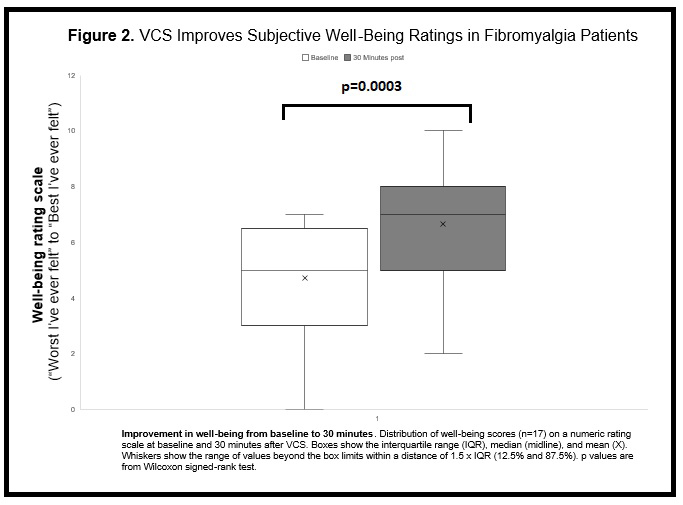
Results: Of the outcome measures examined to date, the immediate effects of VCS are most evident. Overall there was a rapid pain reduction at 5 min post-VCS and, at 30 min, pain had decreased in 14/17 (82.4%) participants, increased in 1/17 (6%) and unchanged in 2/17 (12%) (Figure 1; p = .0003—Wilcoxon signed-rank test). In those with pain reductions, half (7/14) showed an improvement of at least 50% from baseline, 1/14 (7%) improved at least 30% and 6/14 (43%) improved < 30%. Subjective well-being ratings had also improved at 30 min post-VCS in 9/17 (53%) participants, worsened in 3/17 (18%) and were unchanged in 4/17 (24%) (Figure 2; p = .0154—Wilcoxon signed-rank test). While a proportion of participants (8/12 or 67%) reported the procedure was uncomfortable, it was otherwise generally well-tolerated with the large majority (11/12 or 92%) indicating they would have VCS again if it reduced their pain by ≥50% for at least one week — in keeping with recently published VCS tolerability results in a persistent pain cohort (Ngo et al., 2020 Brain Stimul 13: 1446–8). There were also no major adverse events in the current trial. Longer-term VCS effects will be reported when primary outcome data collection is completed, however three patients to date have reported sustained & meaningful pain relief for one week after a single VCS administration.
Conclusion: These preliminary results suggest VCS is a safe & well-tolerated procedure with positive effects on pain & subjective well-being in FM. Further investigation is required with repeated VCS (rVCS) in a placebo-controlled randomized trial.
To cite this abstract in AMA style:
Kaplan M, Zhou C, Carroll E, Weinberg A, Clauw D, Thanh Ngo T, Tassiulas I. Vestibulocortical Stimulation with Caloric Irrigation Reduces Pain and Improves Subjective Well-Being in Fibromyalgia: An Open-Label Pilot Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/vestibulocortical-stimulation-with-caloric-irrigation-reduces-pain-and-improves-subjective-well-being-in-fibromyalgia-an-open-label-pilot-trial/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vestibulocortical-stimulation-with-caloric-irrigation-reduces-pain-and-improves-subjective-well-being-in-fibromyalgia-an-open-label-pilot-trial/