Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
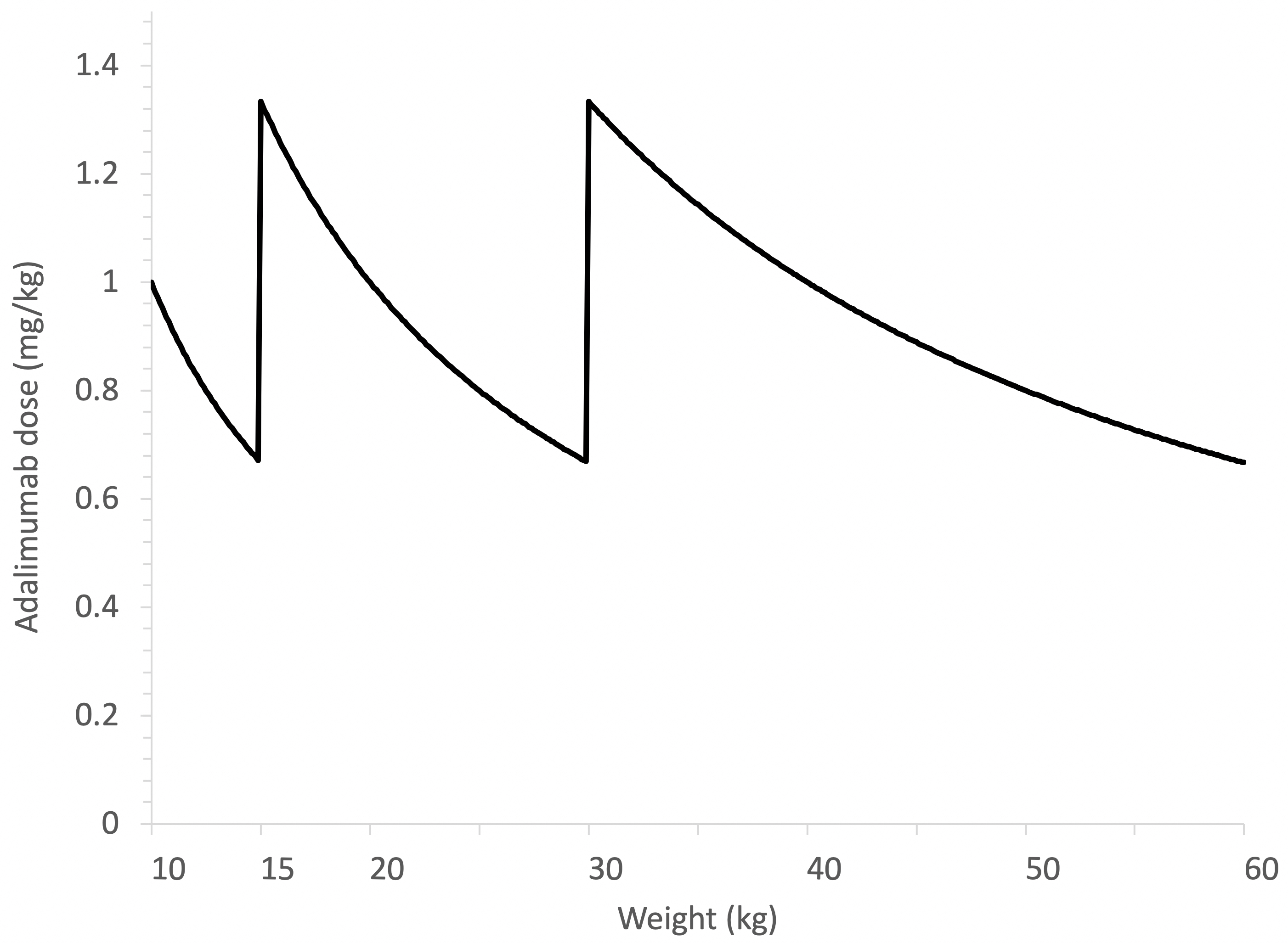
Background/Purpose: Different dosing strategies of adalimumab and etanercept have been used over the past decade in the treatment of juvenile idiopathic arthritis (JIA). With regards to adalimumab, dosing was initially 24 mg/m2 body surface area (BSA) every other week (eow) but changed to fixed dosing by weight categories (10-15 kg: 10 mg eow; 15-30 kg: 20 mg eow; ≥30 kg: 40 mg eow), resulting in large variation in body weight-adjusted exposure (See Figure). For etanercept, the dosing recommendations remained 0.8 mg/kg per week, but the presence of single-dose prefilled syringes (25 or 50 mg) unintentionally promotes rounding of doses. These practices contribute to variable drug exposure in patients with JIA. In addition, metabolism of therapeutic proteins is increased in younger children especially in those aged < 6 years, which reduces drug exposure (i.e., serum drug concentrations). We hypothesize that a higher relative dose (mg/kg or mg/m2) improves the treatment response of children with JIA who start adalimumab or etanercept.
Methods: This is a retrospective cohort study of CARRA Registry data, which contains prospectively collected data from children with rheumatic diseases. Patients aged < 18 years old were included in the analysis if they had a diagnosis of JIA (systemic JIA excluded), started adalimumab or etanercept after Registry enrollment as their first biologic DMARD, and had follow-up data available. The following outcomes were determined between 4-7 months after start of treatment: pediACR100, 90, 70, 50, 30, clinical juvenile arthritis disease activity score (cJADAS; clinically inactive disease: ≤ 1; low disease activity ≤ 2 for oligoarticular course, ≤ 3.8 for polyarticular course), as well as treatment continuation for > 6 months, as a surrogate marker for treatment efficacy. The weight- and BSA-adjusted dose (mg/kg and mg/m2, respectively) were compared using Student’s t-tests (significance p < 0.05; no adjustment for multiple comparisons) between patients with and without the outcome and stratified by age group (< 6 years, 6-12 years, 12-18 years).
Results: This analysis included 406 and 296 patients treated with adalimumab and etanercept, respectively. Children < 6 years old attaining pediACR90/100 received higher adalimumab dosing (1.32 vs 1.06 mg/kg [p = 0.03] and 31.78 vs 25.42 mg/m2 [p = 0.02]). For etanercept, children (all ages) who achieved cJADAS ≤ 1 received lower doses by BSA (24.79 vs 27.53 mg/m2, p=0.01; See Table). In the subgroup analysis, this was only true for 6-12 years (cJADAS ≤ 1: 22.98 vs 27.60 mg/m2 [p = 0.007]; cJADAS ≤ 2: 21.80 vs 29.52 mg/m2 [p = 0.003]). All other analyses did not show associations between dose and outcome variable.
Conclusion: This study provides some evidence that children younger than 6 years old have improved treatment response (pediACR90/100) when treated with a relatively higher dose of adalimumab, while lower etanercept dosing was associated with improved cJADAS outcome. As these analyses are unadjusted, further studies using a Bayesian approach with propensity score matching will follow to adjust for patient characteristics that may contribute to receiving a higher treatment dose (e.g., age, disease activity, uveitis, etc).
 Dose comparison between patients (all ages) who achieved the outcome of interest, and those who did not. BSA, body surface area. Numbers in bold represent the number of patients. Underlined results reached statistical significance (p < 0.05).
Dose comparison between patients (all ages) who achieved the outcome of interest, and those who did not. BSA, body surface area. Numbers in bold represent the number of patients. Underlined results reached statistical significance (p < 0.05).
To cite this abstract in AMA style:
Verstegen R, Shrader P, Balevic S, Beukelman T, Correll C, Dennos A, Phillips T, Feldman B. Variations in Adalimumab and Etanercept Dosing in Juvenile Idiopathic Arthritis and Their Effect on Treatment Outcome: A Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/variations-in-adalimumab-and-etanercept-dosing-in-juvenile-idiopathic-arthritis-and-their-effect-on-treatment-outcome-a-childhood-arthritis-and-rheumatology-research-alliance-carra-registry-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/variations-in-adalimumab-and-etanercept-dosing-in-juvenile-idiopathic-arthritis-and-their-effect-on-treatment-outcome-a-childhood-arthritis-and-rheumatology-research-alliance-carra-registry-study/