Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Voclosporin used in addition to Mycophenolate Mofetil and low dose oral steroids in patients with active Lupus Nephritis (LN) was found to be superior to Placebo in Phase 3, global, double blind, randomized control trial (AURORA 1). Herein we report the post-hoc analysis results of patient reported outcome (PRO) measures Lupus Impact Tracker (LIT), LupusPRO (V1.7) used in this clinical trial. LIT is short 10 item unidimensional, while LupusPRO is multidimensional with 43 items.
Methods: 357 patients with biopsy proven active LN were randomized to receive voclosporin 23.7 mg BID or a placebo, in addition to background standard of care. Primary Outcome of interest was complete renal response (CRR) at week 52. PRO measurements were obtained at baseline, 12-, 24- and 52-weeks using (LIT), LupusPRO and SF36. We evaluated Internal consistency reliability (ICR), Convergent validity (CV) using Cronbach alfa and correlation coefficient. We compared magnitude and direction of changes in LIT and LupusPRO domains at varied time points using SF36 Question 1 of self-reported change in health, where we defined ‘Worse’ as a drop of at least 1 category from baseline and ‘Improved’ being an increase of at least 1 category from baseline. T test was used to make comparisons with a p value ≤0.05 considered significant.
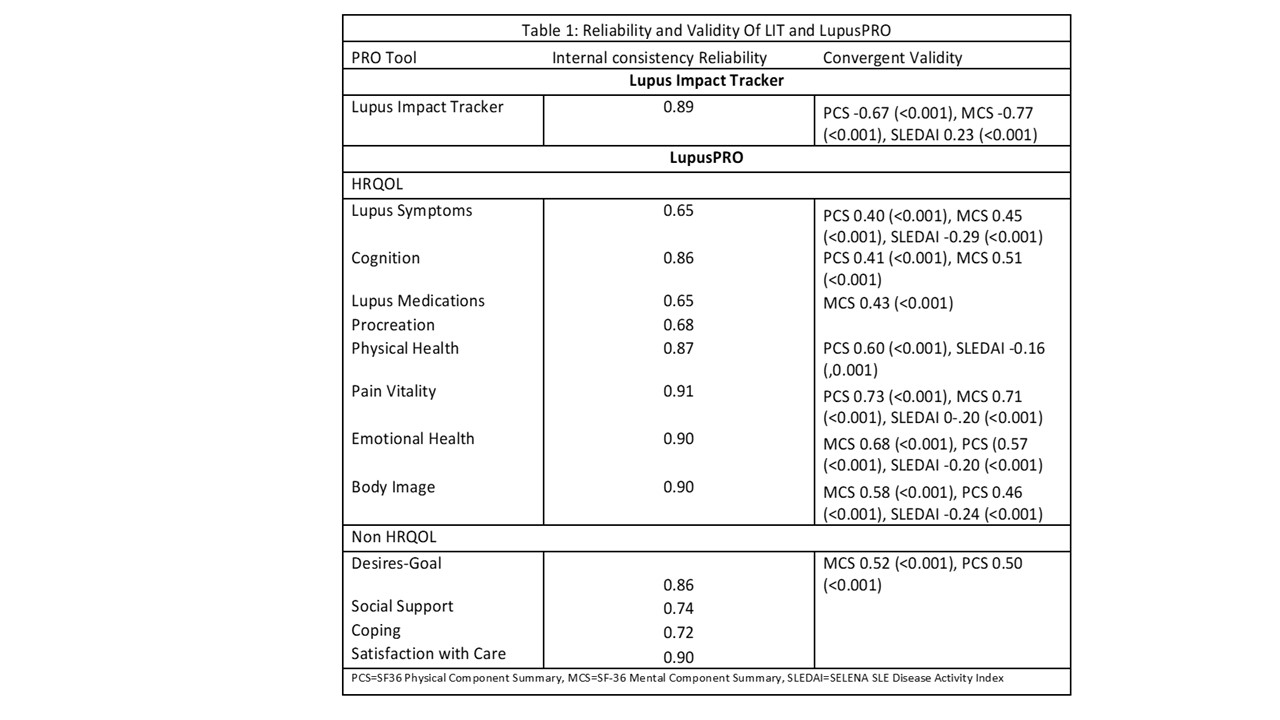
Results: At baseline, ICR of LIT (0.89) and LupusPRO domains (Table 1) were fair. There was good convergent validity of LIT and LupusPRO domains with SF-36 Physical and Mental component summary (PCS & MCS). The three item Lupus Symptom domain had the highest correlation with disease activity (SLEDAI -0.29, p < 0.001).
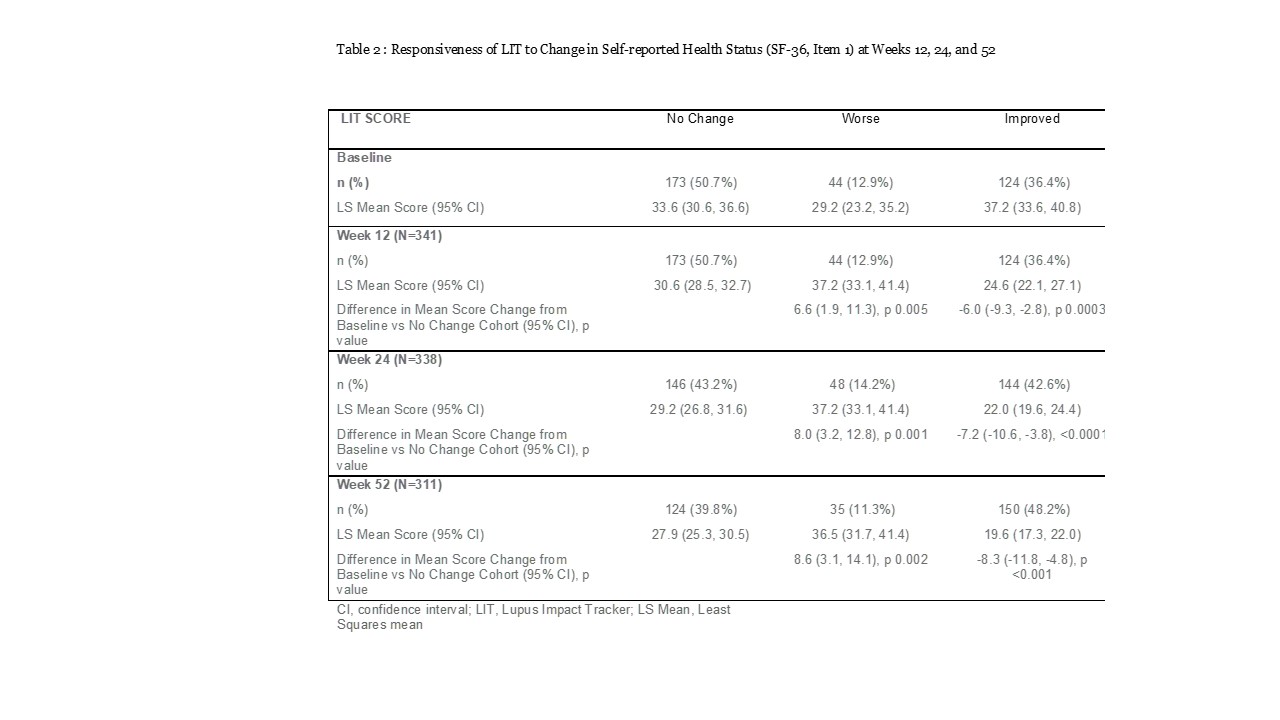
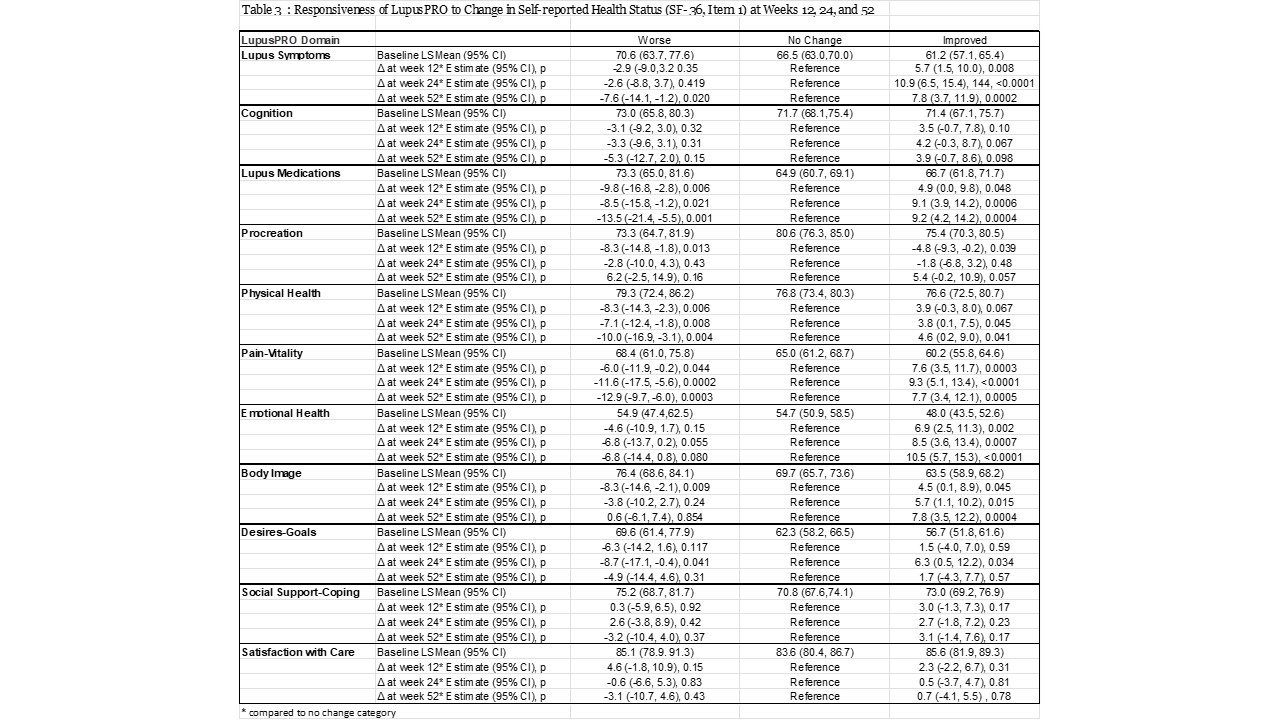
Improvements could be seen as early as 12 weeks. Both LIT and LupusPRO (HRQOL) domains showed responsiveness to change in the appropriate direction at weeks 12, 24 and 52 (Table 2 & 3). LupusPRO HRQOL cognition and procreation domains were less responsive to changes in health; while of the non HRQOL LupusPRO domains, only Desires-Goals domain, showed some responsiveness to change in health at week 24.
Conclusion: Both LIT and LupusPRO show good psychometric properties in this clinical trial, including responsiveness. LIT is short, while LupusPRO is comprehensive. For clinical trials, LIT or LupusPRO HRQOL domains inclusion may be more apt to capture proximal effects of the disease and the proposed intervention. LupusPRO Non HRQOL domains may not change quickly and are more appropriate to evaluate in a routine patient care setting to assess distal impacts of the disease/treatment, available internal and external resources, and their satisfaction with the care.
To cite this abstract in AMA style:
Jolly M, Truman M, Flauto R, Dao K. Validity, Reliability and Responsiveness of Lupus Impact Tracker and LupusPRO: AURORA Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validity-reliability-and-responsiveness-of-lupus-impact-tracker-and-lupuspro-aurora-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validity-reliability-and-responsiveness-of-lupus-impact-tracker-and-lupuspro-aurora-trial/