Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: It is important to assess symptoms of pain, fatigue, anxiety, depression, sleep disturbance, and impaired physical function in patients (pts) with psoriatic arthritis (PsA), as these symptoms are common and can negatively affect health-related quality of life (HRQoL).1 The Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) Profile is a generic health outcomes instrument that is validated in several general and disease-specific populations2 but not in PsA. Here we validate PROMIS-29 Profile psychometric properties using data from the global DISCOVER-1 Phase 3 study of guselkumab vs placebo in 381 pts with active PsA (≥3 swollen & ≥3 tender joints; C-reactive protein ≥0.3 mg/dL; 31% had prior TNF inhibitor exposure) and inadequate response to standard therapies.
Methods: The PROMIS-29 Profile contains 4 items for each of 7 domains (physical function, anxiety, depression, fatigue, sleep disturbance, social participation, pain interference; 28 items scored on 5-point Likert scale) and 1 pain intensity item (0-10 visual analog scale). Raw scores are converted to standard T-scores, with norms based on a general US population mean=50 and SD=10; changes ≥5 points are considered clinically meaningful. PROMIS-29 validation assessments included convergent and discriminant analyses based on Spearman’s correlation (rs) with 36-item Short Form Health Survey (SF-36) domain scores (rs < 0.4=weak, 0.4-< 0.6=moderate, 0.6-1.0=strong correlation); known-groups validity of mean T-scores by the anchor variable of Patient Global Assessment Disease (PGAD) quartile scores at Week (W) 24; and test-retest reliability based on intra-class correlation (ICC) between PROMIS-29 scores at baseline/W16/W24 in pts with stable (change within ±10) PGAD (ICC ≥0.7=acceptable). Analyses were conducted using observed data, combining pts across treatment groups.
Results: The strongest correlations (rs >0.8) between PROMIS-29 and SF-36 domains were observed for concepts that are similar for both instruments (eg, pain interference and pain intensity vs bodily pain; physical function vs physical function; fatigue vs vitality); correlations were weak (rs < 0.4) for dissimilar domains (Table 1). Mean T-scores across subgroups defined by PGAD quartiles at W24 were significantly different for all PROMIS-29 domains, indicating known-groups validity (Figure). ICCs of test-retest reliability in pts with stable (change within ±10) PGAD at W16 and W24 were generally ≥0.7, indicating PROMIS-29 results are reproducible when no change has occurred (Table 2). Mean (SD) PROMIS-29 T-score changes from baseline to W24 were sensitive to PGAD changes in disease severity. In pts with PGAD improvements of 21-30 and 31-40 points, respectively, absolute mean changes across PROMIS-29 domains ranged from 2.7 (anxiety) to 5.4 (pain interference) and 3.1 (sleep disturbance) to 6.6 (pain interference), respectively, from baseline to W24.
Conclusion: This analysis confirms the reliability and validity of the PROMIS-29 Profile to assess HRQoL in pts with active PsA, as well as the responsiveness of this instrument for detecting change.
References
1. Orbai A et al. Ann Rheum Dis. 2017;76:673-80.
2. www.healthmeasures.net.
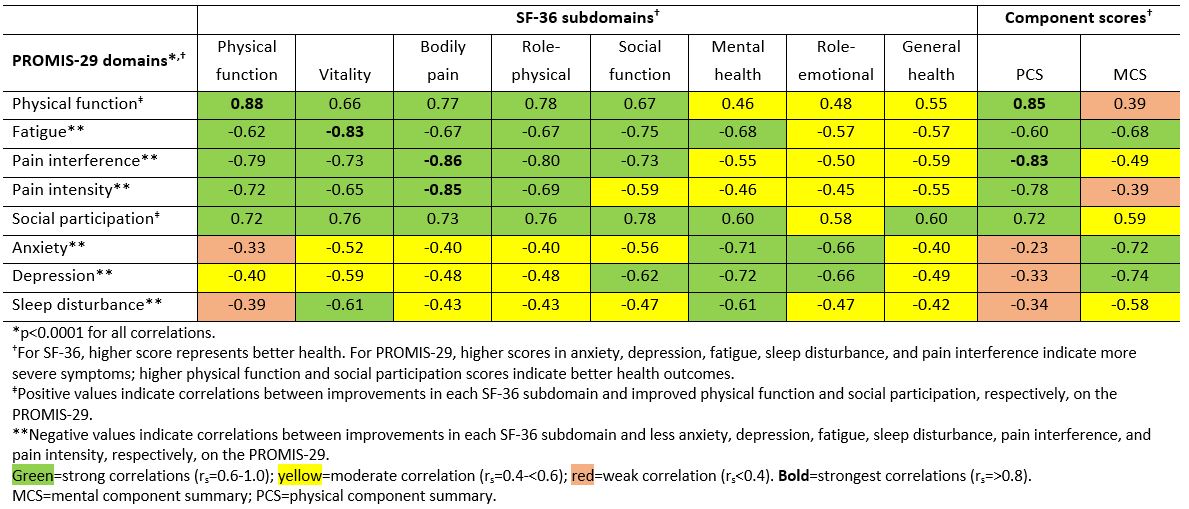 Table 1. Spearman’s correlation between PROMIS_29 domains and SF_36 subdomains and component scores
Table 1. Spearman’s correlation between PROMIS_29 domains and SF_36 subdomains and component scores
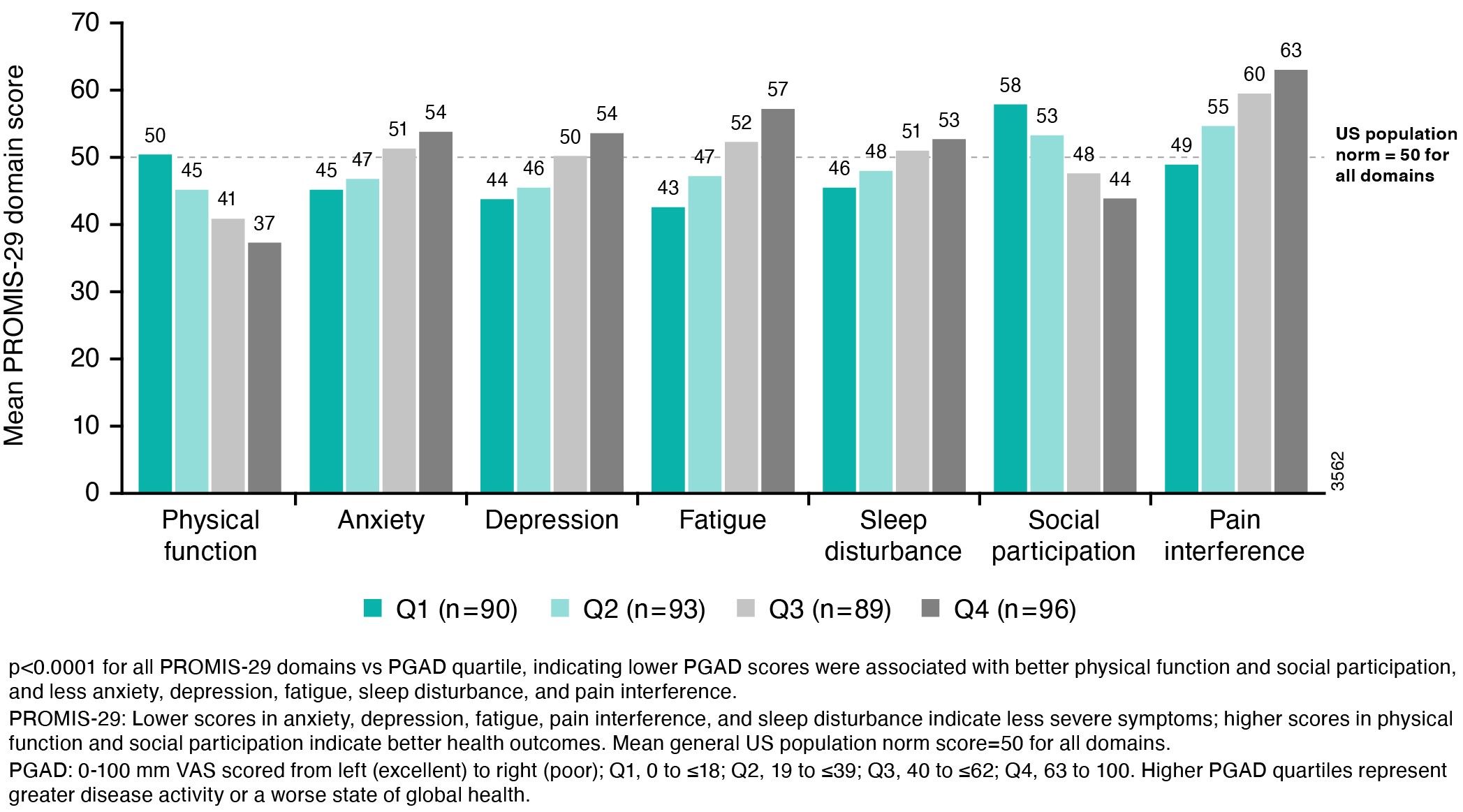 Figure. Mean PROMIS_29 Domain T-Scores by PGAD Quartile at W24
Figure. Mean PROMIS_29 Domain T-Scores by PGAD Quartile at W24
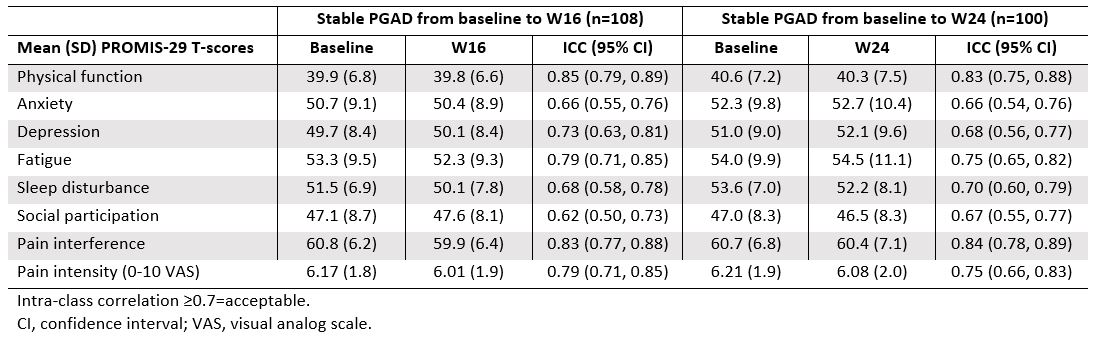 Table 2. Test-retest reliability among pts with stable PGAD (change within ±10 points) over time
Table 2. Test-retest reliability among pts with stable PGAD (change within ±10 points) over time
To cite this abstract in AMA style:
Orbai A, Coates L, Deodhar A, Ritchlin C, Kollmeier A, Neuhold M, Liu Y, Liu Y, Han C. Validation of the PROMIS-29 Profile in Patients with Active Psoriatic Arthritis Using Data from a Phase 3, Randomized, Placebo-Controlled Study Evaluating Guselkumab (TREMFYA®) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/validation-of-the-promis-29-profile-in-patients-with-active-psoriatic-arthritis-using-data-from-a-phase-3-randomized-placebo-controlled-study-evaluating-guselkumab-tremfya/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-promis-29-profile-in-patients-with-active-psoriatic-arthritis-using-data-from-a-phase-3-randomized-placebo-controlled-study-evaluating-guselkumab-tremfya/
