Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The Stanford Health Assessment Questionnaire Disability Index (HAQ-DI) is a widely used patient reported outcome for functional disability in RA. Minimal clinically important differences (MCIDs) have previously been calculated based on the original ordinal HAQ-DI scores resulting in a dependence of the MCIDs on baseline scores(1), causesing limited generalizability and validity of the proposed MCIDs. The Rasch model provides a transformation of ordinal scores to an underlying latent scale with interval scale properties. This requires adequate fit of HAQ-DI data to the model. We aimed to 1) examine internal construct validity of the Danish version of the HAQ-DI (scored without correction for devices) and 2) determine a MCID of the HAQ-DI in a cohort of RA patients initiating anti-rheumatic treatment in clinical practice based on the transformed linear logit scale.
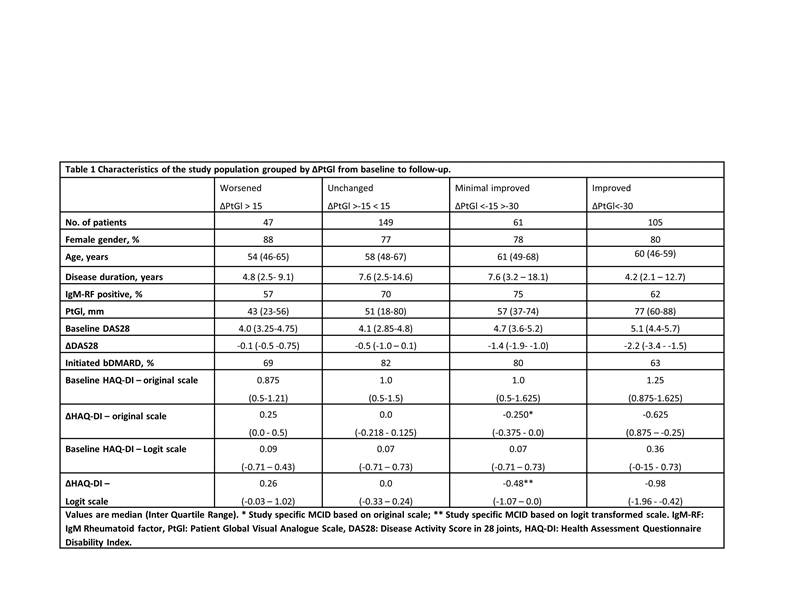
Methods: RA patients registered in the DANBIO registry at Center for Rheumatology and Spine Diseases, Copenhagen, Denmark were included in the study if HAQ-DI and Patient Global Visual Analogue Scores (PtGl) scores were available at a baseline visit and after > three months of follow-up after initiation of a) first synthetic Disease Modifying Anti Rheumatic Drug (sDMARD) (disease duration < 12 months) or 2) first biological drug (disease duration > 12 months). The Rasch model was fitted to HAQ-DI data at baseline and follow-up and item fit evaluated in separate analyses by comparing observed and expected item-restscore correlations (2). MCID was calculated as the median changes of A) the original and B) logit HAQ-DI score in the group of patients who had improved by 15-30 mm (ie. minimal improvement) on a 0-100 mm PtGl scale.
Results: 362 RA patients were included in the study. HAQ-DI data showed acceptable fit to the Rasch model as no significant evidence of misfit was disclosed at baseline and only a single misfitting item (the “Grip” sub-item, p=0.01) was identified at follow-up. Consistent item ranking across time indicated instrument invariance. Changes in ordinal and logit HAQ-DI scores were strongly correlated (Spearmans rho = 0.935, p <0.001), but the association between them is not completely linear. Sixty-one patients had an improvement > MCID on the logit scale (0.48), but no improvement on the ordinal scale (MCID 0.250), while no patients had the opposite pattern.
Conclusion: The Danish version of the HAQ-DI scored without correcting for devices showed acceptable internal construct validity and thus a MCID based on a linear transformed scale could be calculated for this study (0.48). Application of the logit MCID classified an additional 17% of patients as having achieved a MCID compared to the MCID calculated on the ordinal scale. This finding has potential implications for the use of ordinal-based MCID and the powering of future studies. References:
1. Doganay et al.J Rheumatol. 2016 Jan;43(1):194-202
2. Kreiner S. Appl. Psychol. Meas. 2011;35(7):557-561.
To cite this abstract in AMA style:
Ørnbjerg LM, Christensen KB, Tennant A, Lund Hetland M. Validation of the Danish Version of the Stanford Health Assessment Questionnaire Disability Index and Determination of the Minimal Clinically Important Difference in a Cohort of Rheumatoid Arthritis Patients Using the Rasch Measurement Model [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/validation-of-the-danish-version-of-the-stanford-health-assessment-questionnaire-disability-index-and-determination-of-the-minimal-clinically-important-difference-in-a-cohort-of-rheumatoid-arthritis-p/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-danish-version-of-the-stanford-health-assessment-questionnaire-disability-index-and-determination-of-the-minimal-clinically-important-difference-in-a-cohort-of-rheumatoid-arthritis-p/