Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: According to international recommendations, the Ankylosing Spondylitis Disease Activity Score (ASDAS) is the preferred score for assessing disease activity in axial spondyloarthritis (axSpA) [1]. However, routine determination of C-reactive protein (CRP) to calculate ASDAS values takes hours to days. This limits the use of ASDAS in clinical routine and clinical trials and hinders the implementation of treat-to-target approaches in axSpA. Whereas quick quantitative CRP (qCRP) tests allow CRP assessment within a few minutes. In a pilot project the performance of qCRP-based ASDAS assessment (ASDAS-qCRP) was already investigated in a single center study of 50 newly diagnosed, bDMARD-naïve axSpA patients with promising results [2]. Therefore, the objective of our study was to validate the ASDAS-qCRP in a prospective, multicenter study of axSpA patients in a typical axSpA cohort with an appropriate sample size.
Methods: The study was conducted in five centers in Germany. Consecutive adult (≥ 18 years) axSpA patients were included. In addition to a rheumatological assessment, including patient reported outcomes (PROs), routine CRP and erythrocyte sedimentation rate (ESR) were measured in the local labs. Additionally, a qCRP testing with the „QuikRead go instrument“ (Aidian Oy, Finland) was performed at the study center (measurement range 0.5 – 200 mg/l for hematocrit concentrations of 40 – 45%). Statistical analysis included descriptive statistics, cross tabulation and weighted Cohen´s kappa comparing disease activity categories, Bland-Altman plots and intraclass correlation coefficient (ICC) for ASDAS-CRP and ASDAS-qCRP.
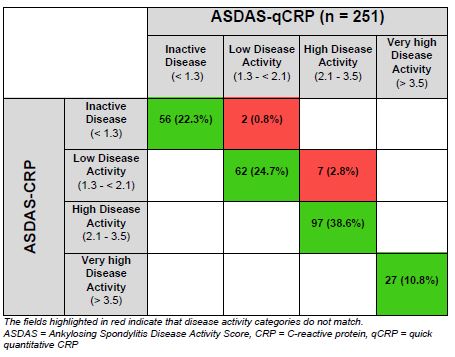
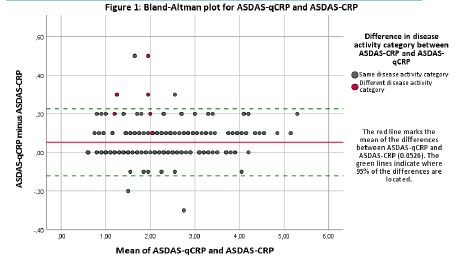
Results: In this study 251 axSpA patients were included between January and September 2020 (mean age: 38.4 years; mean disease duration: 6.2 years, 159 patients (63.3%) were male, 211 (84.1%) HLA-B27 positive and 195 (77.7%) were classified as radiographic axSpA). 143 patients (57.0%) were treated with bDMARDs. CRP and qCRP showed mean values of 2.12 and 2.17 mg/l, respectively. With the ASDAS-qCRP, 242 patients (96.4%) were assigned to the same disease activity category as compared to the ASDAS based on the conventional lab CRP measurement (Table 1). Weighted Cohen´s kappa was 0.966 (95%CI: 0.943; 0.988). ICC for ASDAS-CRP- and ASDAS-qCRP-values was 0.997 (95%CI: 0.994; 0.999). The agreement of ASDAS-qCRP and ASDAS-CRP is shown in a Bland-Altman plot (Figure 1).
Conclusion: The ASDAS-qCRP and ASDAS-CRP showed an almost perfect agreement on the assignment to disease activity categories (96%) with the important advantage of time. With ASDAS-qCRP, rheumatologists could base their clinical decision-making on a disease activity measurement by using a composite score immediately. ASDAS-qCRP, therefore, can be integrated in clinical routine and clinical trials in the future and may facilitate implementation of the treat-to-target concept in axial SpA.
References:
1. Smolen JS, et al. Ann Rheum Dis. 2018 Jan; 77(1):3-17.
2. Proft F, et al. Joint Bone Spine. 2019 Jul 29.
Acknowledgements: The authors would like to deeply thank Braun T, Doerwald C, Deter N, Höppner C, Lackinger J, Lorenz C, Lunkwitz K, Mandt B, Sron S and Zernicke J for their practical support and coordinating the study.
To cite this abstract in AMA style:
Proft F, Schally J, Brandt H, Brandt-Jrgens J, Burmester G, Haibel H, Käding H, Karberg K, Lüders S, Muche B, Protopopov M, Rademacher J, Rios Rodriguez V, Torgutalp M, Verba M, Zinke S, Poddubnyy D. Validation of the Ankylosing Spondylitis Disease Activity Score with a Quick Quantitative Creactiveprotein Assay (ASDAS-qCRP) in Patients with Axial Spondyloarthritis (axSpA): Aprospective, National, Multicenter Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/validation-of-the-ankylosing-spondylitis-disease-activity-score-with-a-quick-quantitative-creactiveprotein-assay-asdas-qcrp-in-patients-with-axial-spondyloarthritis-axspa-aprospective-national/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-the-ankylosing-spondylitis-disease-activity-score-with-a-quick-quantitative-creactiveprotein-assay-asdas-qcrp-in-patients-with-axial-spondyloarthritis-axspa-aprospective-national/