Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: In systemic lupus erythematosus (SLE), there is not a standardized and validated definition of states of moderate and severe SLE activity. The aim of our study is to propose a definition for moderate disease activity state (MODAS) and severe disease activity state (SEDAS) in SLE and using the RELESSER-PROS cohort to describe the prevalence of both states and to analyze the impact of this categorization on different outcomes
Methods: The study population belongs to the RELESSER-PROS prospective cohort with data from patients followed annually for 4 years. The MODAS and SEDAS definitions were generated by a panel of lupus experts, which may be differentiated depending on absolute score of clinical Systemic Lupus Erythematosus Disease Activity Index (cSLEDAI), certain clinical manifestations not considered in SLEDAI and the subjective assessment of the physician, through PGA. MODAS was defined as the presence of at least one of the following conditions: < 4 cSLEDAI < 8 or 1< PGA < 2 (without severe clinical manifestations); and SEDAS: SLEDAIc >8 or PGA > 2 or the presence of severe SLEDAI and Non-SLEDAI manifestations. Low disease activity (No MODAS nor SEDAS): 1<SLEDAIc <4 or 0< PGA<1. Subsequently, an internal validation was carried out, analyzing the impact of belonging to one of the predefined groups in several robust outcomes overtime. For this purpose, the mortality, damage accrual (using the SLICC/ACR Damage Index [SDI]), number of severe flares (SFI definition), hospital admissions and HRQoL according to the Lupus Impact Tracker (LIT), were collected at each visit.
A descriptive analysis was conducted for the variables based on the presence of MODAS or SEDAS in at least 1 of the 5 visits and according to the number of visits with MODAS or SEDAS. The significance level for all tests was set at 0.05.
Results: A total of 1463 patients (90% women) were included, with a mean age (±SD) of 56 (±13.5) years. The mean disease duration of SLE (±SD) at V1 was 14 (±8.5) years.
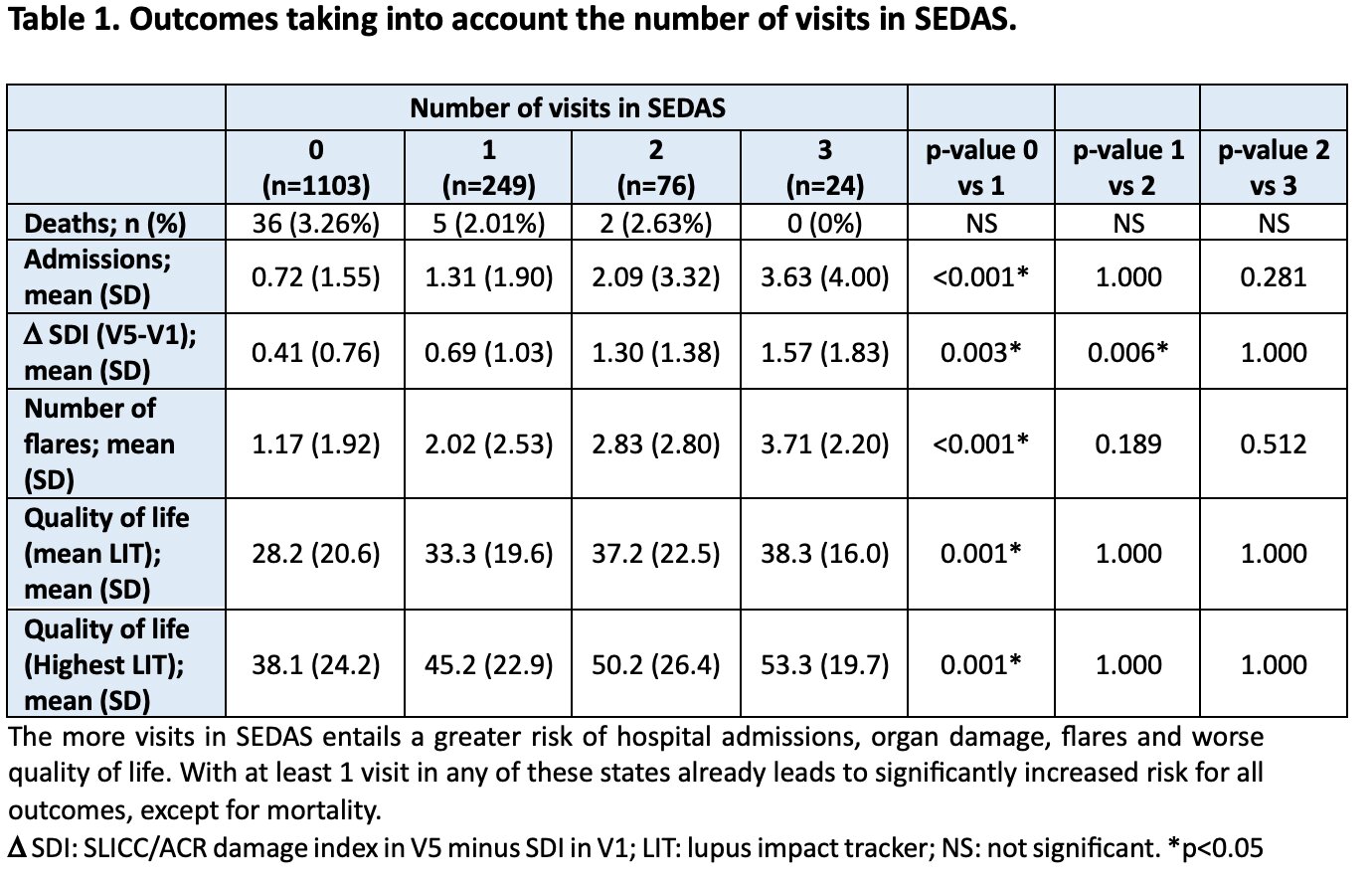
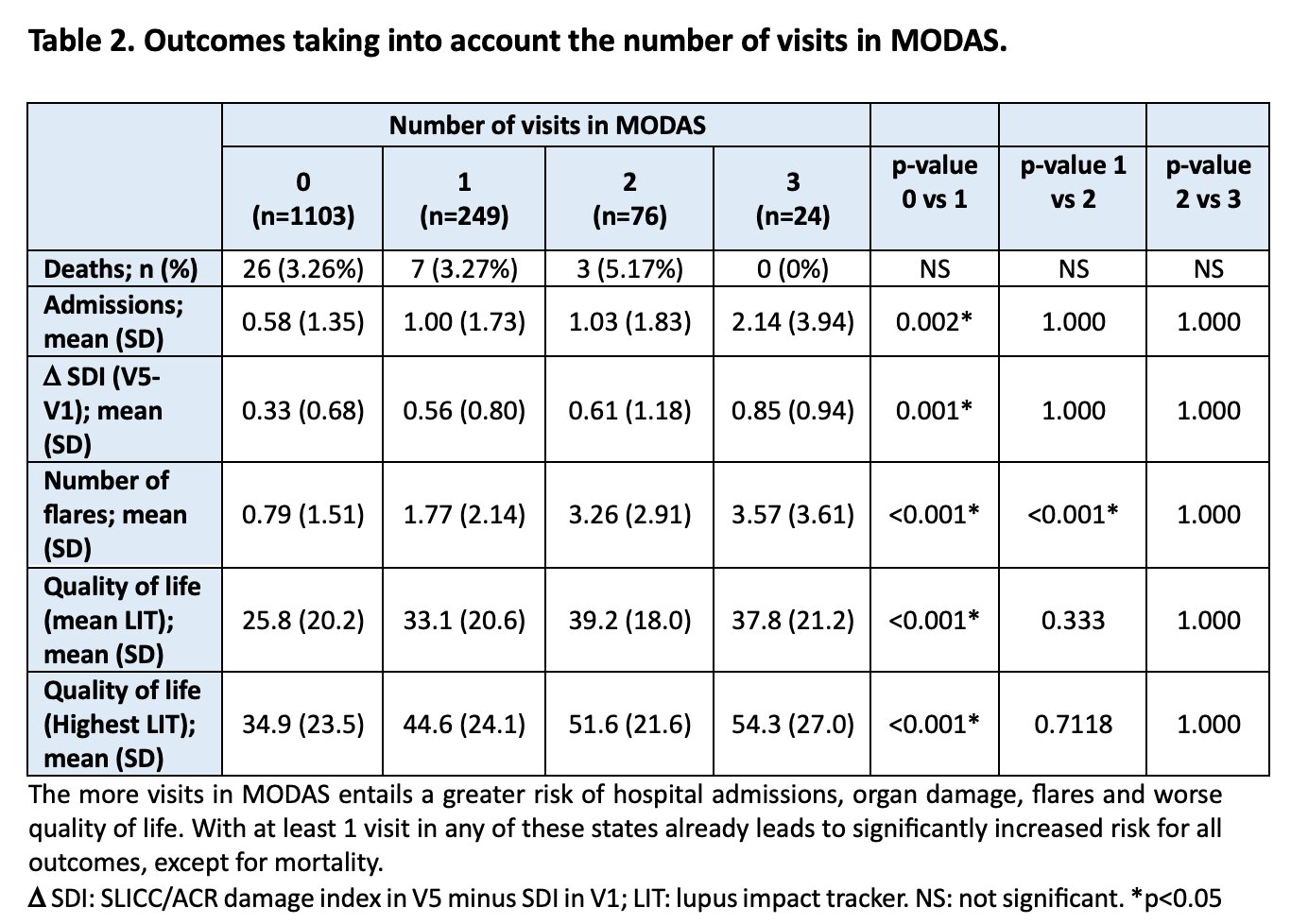
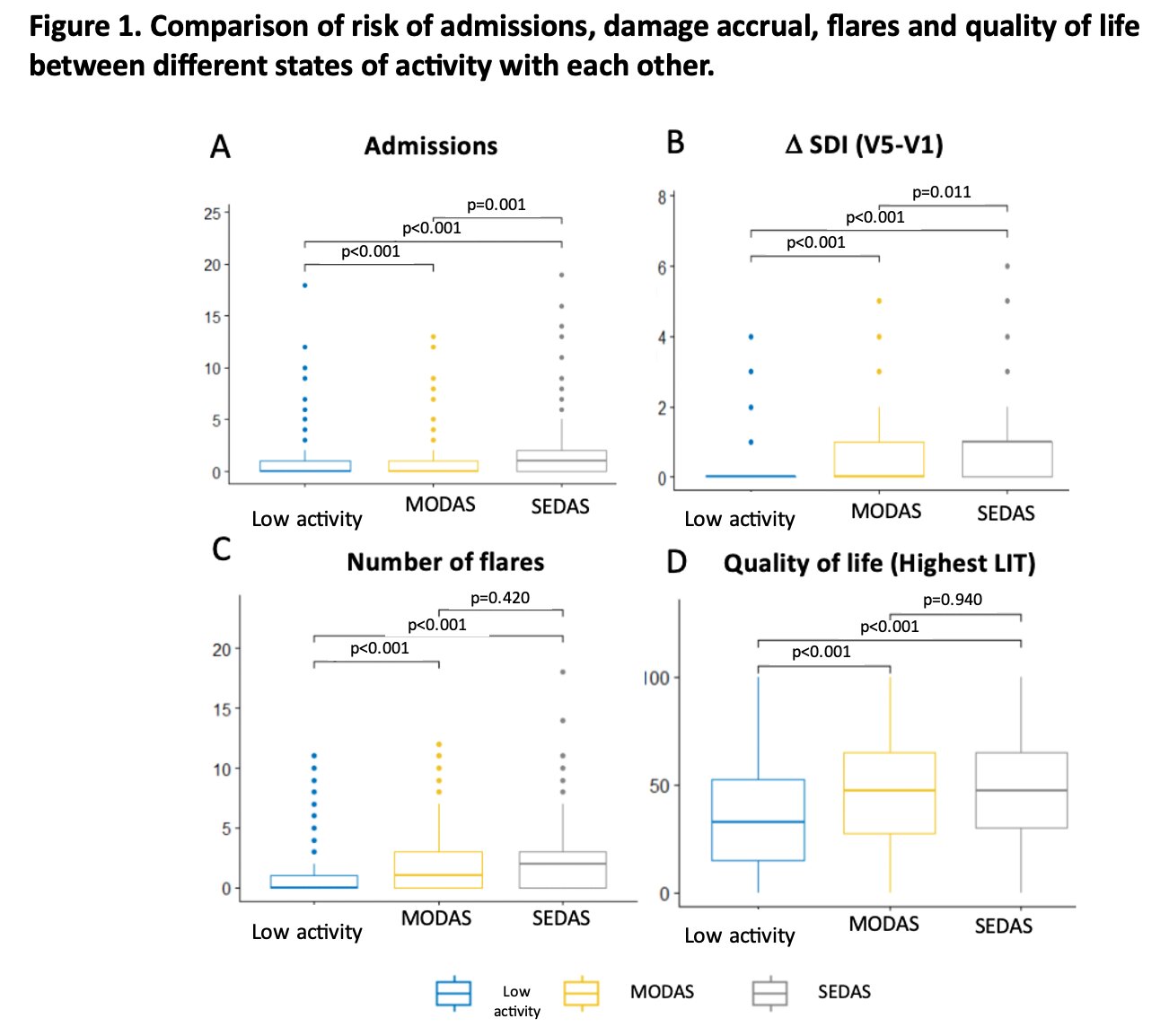
Patients with at least 1 visit in MODAS or in SEDAS had significantly most damage accrual higher number of flares, more hospital admissions, and worse quality of life (p< 0.001). Damage accrual and hospital admissions were significantly higher in SEDAS than in MODAS. This is also the case for all the outcomes, except for mortality, when comparing being in MODAS or SEDAS with not being in these states. Figure 1. Besides, the more visits in MODAS or in SEDAS, greater damage, more admissions, more flares and worse quality of life. The SEDAS entails the greater risk of all the defined activity states. Table 1 and 2.
Conclusion: Patients who were in a state of moderate or severe activity at least one time had worse outcomes at the end of follow-up in terms of damage accrual, hospital admissions, number of flares and deterioration in HRQoL. Furthermore, more visits in these states entail a greater risk. The SEDAS is the one that is related to the worst outcomes. These results emphasize the importance of an adequate stratification of disease activity in SLE patients.
To cite this abstract in AMA style:
Altabás-González I, Rúa-Figueroa Í, Mouriño C, Mamani I, Roberts K, Martínez-Barrio J, Galindo-Izquierdo M, Calvo-Alen J, ERAUSQUIN ARRUABARRENA M, Serrano-Benavente B, Carrión-Barberà I, Uriarte-Isacelaya E, Tomero Muriel E, Freire González M, Blanco-Alonso R, Salgado-Pérez E, Vela-Casasempere P, Fernández-Nebro A, Olive A, Sanguesa C, Narvaez-García J, Menor Almagro R, Rosas-Gómez de Salazar J, Hernández Beriain J, Manero-Ruiz F, Aurrecoechea E, Montilla C, Bonilla g, Ibarguengoitia Barrena O, Torrente-Segarra V, Cacheda A, García-Villanueva M, Moriano C, Horcada L, Lozano-rivas N, Bohorquez C, Pego-Reigosa J. Validation of Proposals for Definitions of Moderate and Severe Disease Activity in Systemic Lupus Erythematosus, Based on Data Gathered from the RELESSER-PROS Register Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validation-of-proposals-for-definitions-of-moderate-and-severe-disease-activity-in-systemic-lupus-erythematosus-based-on-data-gathered-from-the-relesser-pros-register-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-proposals-for-definitions-of-moderate-and-severe-disease-activity-in-systemic-lupus-erythematosus-based-on-data-gathered-from-the-relesser-pros-register-database/