Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (SJIA) involves dysregulation of inflammation and innate immunity, and can cause life-threatening complications including lung disease (LD). However, there are no validated patient-reported outcomes (PRO) that measure SJIA-LD. The primary aims are to validate a lung symptom survey created by Cincinnati Children’s Medical Health Center (survey A), and one based on existing Patient-Reported Outcomes Measurement Information System (PROMIS) measures (survey B). We hypothesize that patients with LD have higher scores compared to those without LD.
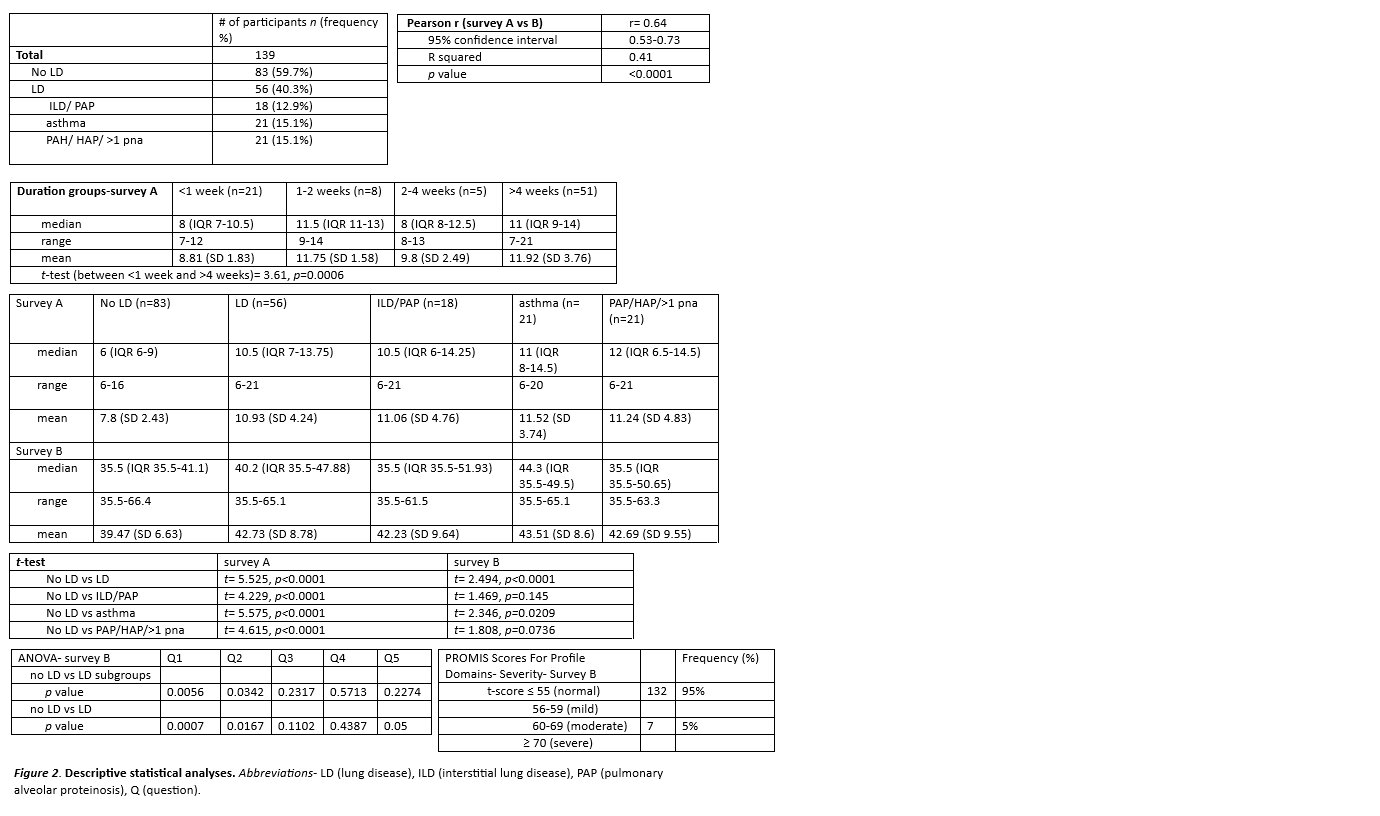
Methods: Parents or guardians of SJIA patients under 18 years of age, as identified through the CCHMC JIA database and Systemic JIA Foundation, were surveyed via mail or email, and responses stored in Research Electronic Data Capture. LD was divided into three subgroups of patients: interstitial lung disease (ILD) or pulmonary alveolar proteinosis (PAP); asthma; and pulmonary hypertension (PAH), hospital-acquired pneumonia (HAP), or recurrent pneumonia. Total scores were used for survey A. PROMIS survey B are represented by t-scores, defined as standardized scores with a mean of 50 and a standard deviation of 10. Correlation between surveys were made with Pearson coefficient, while between-survey comparisons were done with t-tests, and between-group comparisons with ANOVA. Severity for lung symptoms in survey B were determined by the PROMIS Scores Graph For Profile Domains.
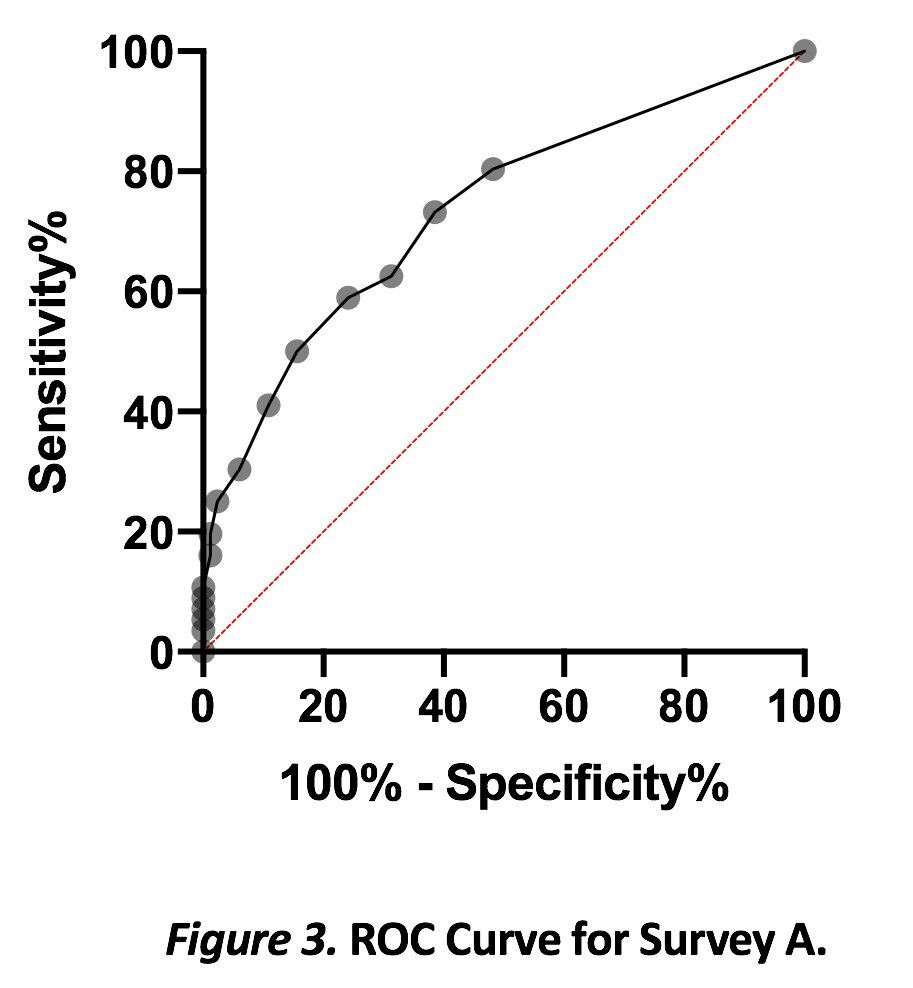
Results: Out of 139 participants, 40.3% (n=56) reported at least one LD including 12.9% (n=18) with ILD or PAP; 15.1% (n=21) with asthma; and 15.1% (n=21) with PAH, HAP, or recurrent pneumonia. There was high correlation (r=0.64) between both surveys. In survey A, the < 1 week group (n=21) had a median of 8 (IQR 7-10.5), and >4 weeks (n=51) had a median of 11 (IQR 9-14; p=0.0006). All patients with LD had significantly higher total scores than patients without known LD, with ROC curve showing an area of 0.73. A total score of >6.5 (range 6-22) had moderate sensitivity of 80.4% but poor specificity of 51.8% for identifying LD. However, patients with ILD and PAP had similar scores to those with asthma.
For survey B, patients with LD had significantly higher scores than patients without LD, although the median score was in the normal range. Further evaluation of LD subgroups showed that survey B was only significant for asthma, likely as the survey was based on existing asthma measures. For lung symptom clinical severity t-score thresholds, 95% was normal and 5% was moderate. The questions for survey B were further scrutinized; questions 1, 2, and 5 were significant when comparing no LD vs LD. Question 1 and 2 were significant when comparing no LD and all lung subgroups. The significant range differences between the questions can be helpful in creating future surveys with meaningful change.
Conclusion: While PROs based on lung disease symptoms showed higher scores in children with SJIA and LD, they could not discriminate between asthma and ILD/PAP. This is in agreement with prior work that most patients with SJIA and LD have mild respiratory symptoms. Future goals would be to identify other patient-reported factors that correlate stronger with LD.
To cite this abstract in AMA style:
Nguyen K, Towe C, Yasin S, Grom A, Brunner H, Schulert G. Validation of Patient-reported Outcomes (PRO) Lung Questionnaires for Systemic Juvenile Idiopathic Arthritis (SJIA) Patients at Risk for Lung Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/validation-of-patient-reported-outcomes-pro-lung-questionnaires-for-systemic-juvenile-idiopathic-arthritis-sjia-patients-at-risk-for-lung-disease/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-patient-reported-outcomes-pro-lung-questionnaires-for-systemic-juvenile-idiopathic-arthritis-sjia-patients-at-risk-for-lung-disease/