Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Administrative claims databases are valuable tools for studying treatment effects in large JIA populations but do not contain direct measures of disease activity, limiting conclusions about treatment effectiveness. We sought to validate a claim-based algorithm based on new medication use as a proxy for worsening JIA disease activity.
Methods: We queried clinical and prescription data in electronic medical records from 3 participating centers (2004-2019) for patients with JIA age < 21. We randomly selected a group of subjects who met a prespecified definition for new antirheumatic drug: 1) newly prescribed conventional or biologic DMARD ≥90 days after diagnosis and ≥90 days after starting the most recent DMARD; 2) DMARD re-initiation ≥180 days since the end of the most recent prescription; 3) systemic steroid prescribed ≥180 days since the most recent prescribed systemic steroid; or 4) intra-articular glucocorticoid injection (IAGC) ≥180 days after the most recent injection and after starting the most recently prescribed JIA drug. One eligible new antirheumatic drug event was randomly selected per subject. We excluded subjects for prior diagnoses of inflammatory bowel disease, immunodeficiency, and non-JIA systemic rheumatic disease (e.g., lupus). We estimated the positive predictive value (PPV) based on the proportion of subjects for whom new antirheumatic drug use was accompanied by documentation of increased disease activity within the prior 90 days. Increased disease activity was defined as: worsening joint symptoms (degree or extent of pain, stiffness, or swelling), new or recurrent joints with active arthritis on exam, new or worsening uveitis, new or recurrent symptoms or signs of systemic inflammation (e.g., fever, rash; systemic JIA only), or worsening disease activity per treating rheumatologist. We also examined the PPV of alternate definitions of new antirheumatic drug use.
Results: 87 subjects were included (Table 1). Of 66 subjects with use of a new conventional or biologic DMARD, 35 (53%) had taken a prior DMARD, and 9 (13.6%) were restarting the same DMARD that was previously stopped. Overall, among subjects who used a new antirheumatic drug, 75% (95% CI 65%, 83%) had evidence of worsening disease activity within the prior 3 months (Table 2). The PPV was lower for those starting a new DMARD (65%) than for those restarting a prior DMARD (89%), starting systemic steroids (91%), or receiving IAGC (93%). The PPV increased with longer durations of time since the last new medicine was prescribed and with longer times after diagnosis (Table 2). Reasons for new prescriptions in subjects without worsening disease activity were: inadequate response to prior treatment (86%), intolerance or side effects (23%), and patient/family preference (5%) (not mutually exclusive).
Conclusion: New prescriptions of antirheumatic drugs may serve as a reasonable proxy for worsening JIA disease activity in claims data. Restricting new use events to at least 6 months after the last newly prescribed drug or after JIA diagnosis may improve the likelihood that these events correspond to worsening disease activity.
 DMARD disease modifying antirheumatic drug, IQR interquartile range, RF rheumatoid factor 1 Some subjects were started on more than 1 drug at the index date
DMARD disease modifying antirheumatic drug, IQR interquartile range, RF rheumatoid factor 1 Some subjects were started on more than 1 drug at the index date
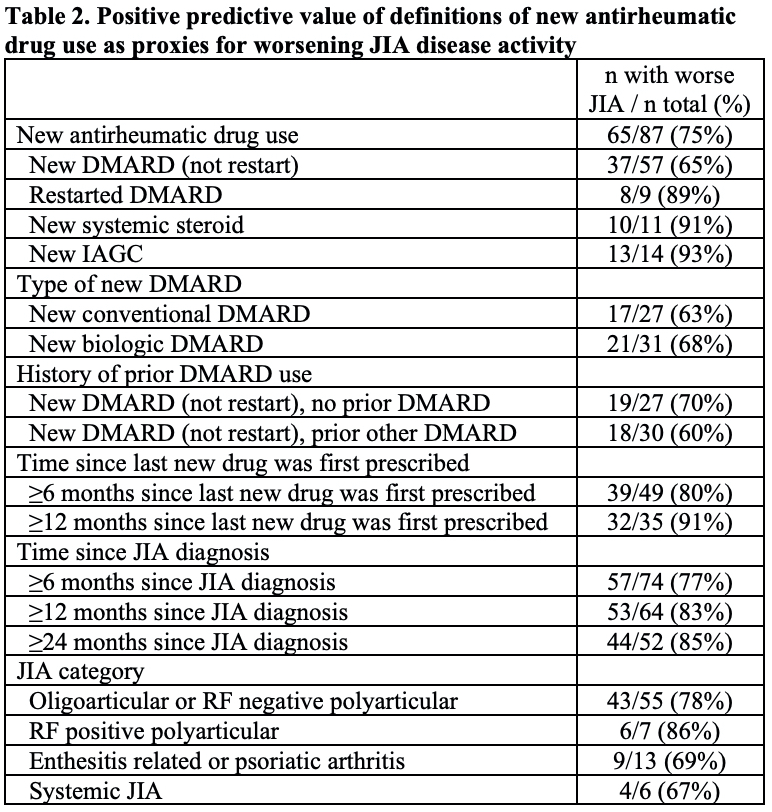 DMARD disease modifying antirheumatic drug, IAGC intra-articular glucocorticoid injection, JIA juvenile idiopathic arthritis, RF rheumatoid factor
DMARD disease modifying antirheumatic drug, IAGC intra-articular glucocorticoid injection, JIA juvenile idiopathic arthritis, RF rheumatoid factor
To cite this abstract in AMA style:
Gabbeta A, Mulvihill E, Beukelman T, Lewis J, Rose C, Strom B, Horton D. Validation of New Antirheumatic Drug Use as a Proxy for Increased JIA Disease Activity [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/validation-of-new-antirheumatic-drug-use-as-a-proxy-for-increased-jia-disease-activity/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-new-antirheumatic-drug-use-as-a-proxy-for-increased-jia-disease-activity/
