Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Herpes zoster (HZ) is a known serious infectious complication in children with autoimmune/autoinflammatory disease and potentially exacerbated by immunomodulatory medications. Information on HZ burden is especially pertinent as JAK-STAT inhibitors have been recently FDA approved for use in children and are known to reactive latent VZV infection. However, our knowledge of HZ prevalence among children is limited. Because of its relative rarity, validating the diagnosis of HZ in healthcare claims and electronic medical record (EMR) databases is a critical first step. This study aimed to evaluate the validity of International Classification of Diseases, 10th Revision, (ICD-10) code-based algorithms for identification of HZ.
Methods: We identified all patients with ICD-10 for HZ (B02.xx) as their primary or secondary diagnosis in the EMR of a large, integrated pediatric healthcare network including 31 primary care practices, 4 urgent care centers, 19 subspecialty divisions, and a 600-bed tertiary care hospital from January 2010 – March 2019. Both ambulatory and inpatient databases were queried. Electronic charts were reviewed to adjudicate diagnostic code-based algorithms for true HZ infection. Positive predictive values (PPVs) for confirmed HZ as well as those with confirmed or possible HZ were calculated for each algorithm.
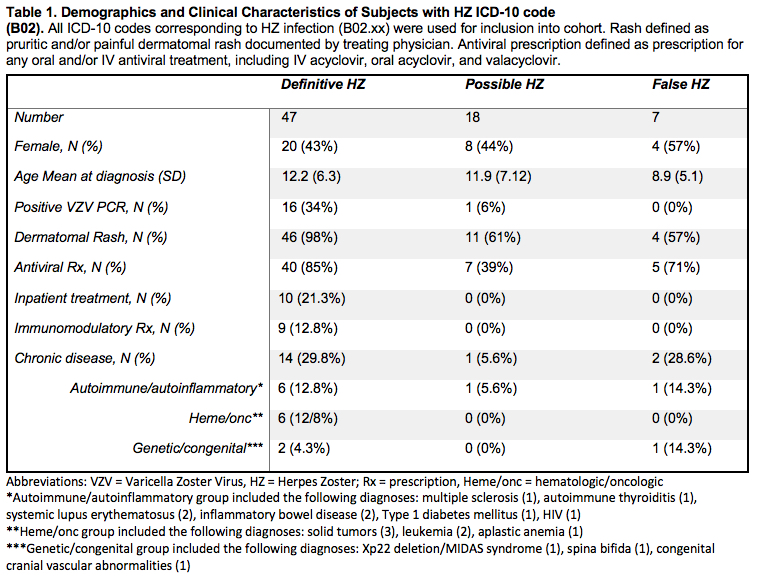
Results: A total of 72 unique individuals were identified as having at least one physician-entered ICD-10 corresponding to HZ (B02). The PPV for a single ICD-10 code was 90.3% (95% CI 80.8-95.4) for definitive and/or possible cases of HZ and 65.3% (95% CI 53.4-75.5%) for definitive cases alone. Adding a prescription for an antiviral did not improve the PPV (90.4%; 78.5-96.0), while adding documentation of pruritic and/or painful dermatomal rash increased the PPV to 93.4 (95% CI 83.5-97.6). The combination of diagnostic code, antiviral prescription and documentation of rash had the highest PPV of 93.5% (95% CI 81.2-97.3) but with substantial loss in sample size. Seventeen of the 72 patients identified (23.6%) had underlying chronic conditions (excluding asthma) requiring frequent follow-up with a sub-specialist. Eight patients (11.1%) had autoimmune/autoinflammatory diagnoses, 6 (8.3%) had oncologic/hematologic diagnoses requiring systemic therapy and 3 (4.2%) had genetic/congenital disease. Out of the 47 cases of definitive HZ, 10 (21.3%) required inpatient hospitalization for IV antiviral therapy, 7 (70%) of which had underlying chronic disease and 5 (50%) of which were on systemic immunomodulatory therapy. Seven out of the 17 patients (41.7%) with chronic disease required inpatient admission for HZ treatment, and 5 of these 7 (71.4%) were on systemic immunomodulatory therapy.
Conclusion: HZ can be identified with a high PPV in electronic medical records of children. There were modest improvements in PPV with the addition of characteristic rash. These findings can be employed in pharmacoepidemiologic research to better understand risk factors for HZ infection, which is especially important as new immunomodulatory medications come to market.
To cite this abstract in AMA style:
Rutstein B, Gmuca S, Gerber J, Ogdie A. Validation of Healthcare Claims Algorithms for Identification of Herpes Zoster Among Children with Autoimmune/Autoinflammatory Disease [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/validation-of-healthcare-claims-algorithms-for-identification-of-herpes-zoster-among-children-with-autoimmune-autoinflammatory-disease/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-healthcare-claims-algorithms-for-identification-of-herpes-zoster-among-children-with-autoimmune-autoinflammatory-disease/