Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Ultrasonography (US) has experienced a rapid evolution in rheumatology. Despite many advantages being repeatedly shown, several barriers persist, equipment cost being an important one. Hand-held US technology promises to substantially lower this cost. However, before it can be used explicitly for rheumatology, its performance must be validated against gold-standard devices. We aim to test the concurrent validity of a handheld US device versus a gold-standard device to detect characteristic features of enthesitis.
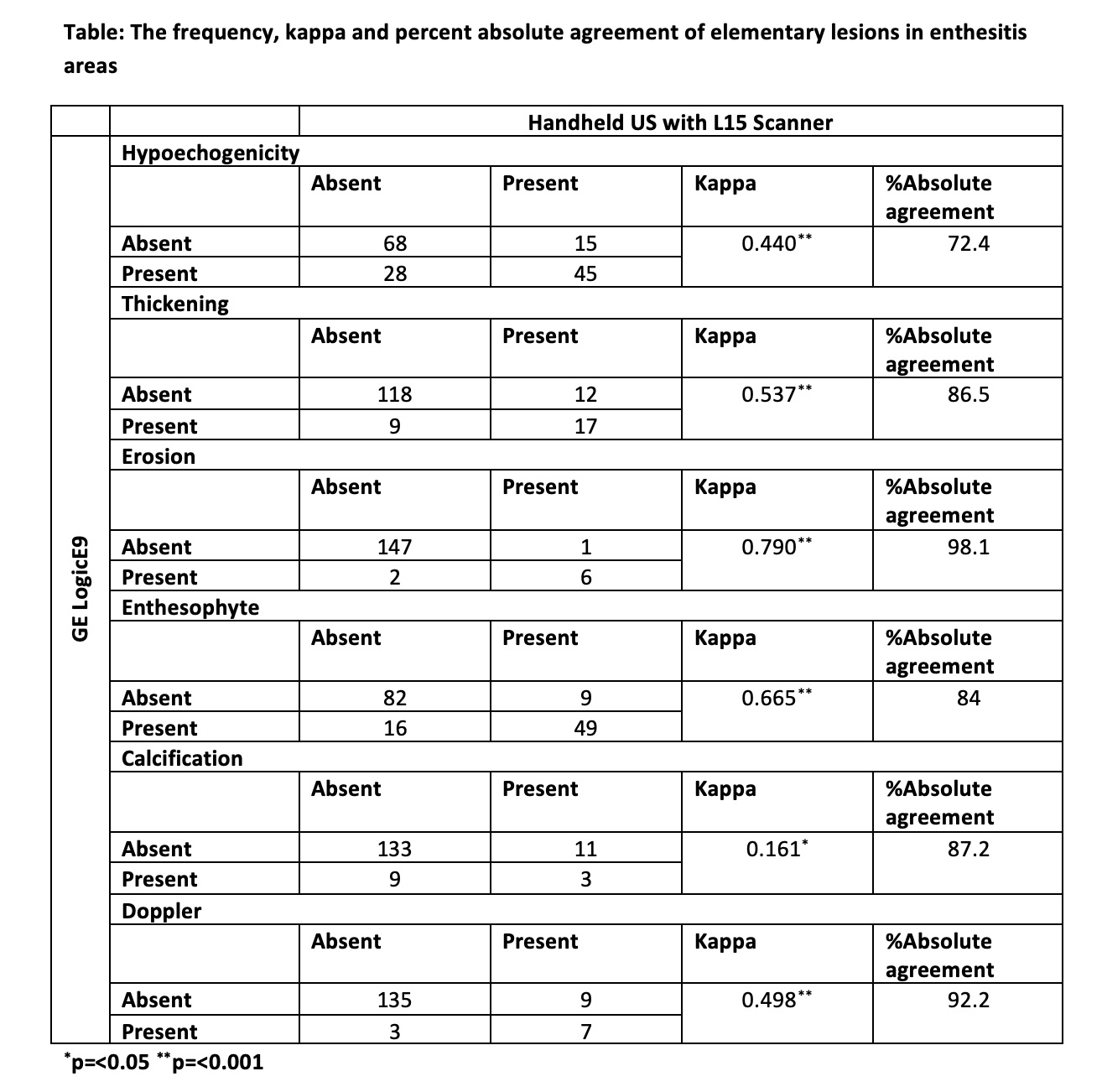
Methods: Peripheral PsA patients with at least one tender and swollen joint were included. Each patient had consecutive US examinations using a handheld (Clarius Mobile Health Inc, HD3 L15 scanner) and a gold-standard US device (GE LogicE9) for detecting elementary lesions of enthesitis (hypoechogenicity, thickening, erosion, enthesophyte, calcification and Doppler). Supraspinatus, triceps, common extensor tendon, quadriceps, patellar ligament (origin and insertion), Achilles tendon and plantar fascia entheses were evaluated. B-mode and power Doppler images were saved for each site and lesion. Every image was given a unique identifier number after collection for blinding purpose. Image reading was performed at least 2 weeks after the acquisition of US in all patients. A random order slide show was conducted for scoring, irrespective of the machine, anatomical site or patient, to ensure blindness. Scoring was done using previously validated methods. The inflammation score was obtained by summing hypoechogenicity, thickening, and Doppler scores, and the chronicity score was obtained by summing erosion, enthesophyte, and calcification scores. The total enthesitis score was determined by the sum of the inflammation and chronicity scores. Cohen’s kappa coefficient was calculated to test the agreement for elementary lesions of enthesitis, in addition to intraclass correlation analysis. Here we present interim analysis for the first 10 patients to detect enthesitis in 160 entheses.
Results: On the day of the US, 8 (80%) patients had at least one tender enthesis on physical examination. The median (IQR) SPARCC enthesitis score was 3(0.75-5.0). The agreement was substantial (see table) for enthesophytes and erosions; moderate for thickening, Doppler and hypoechogenicity; and slight for calcification (table). A very strong agreement was detected between the devices both for the inflammation, chronicity and total enthesitis scores (ICC (intraclass correlation) and p values: inflammation: 0.957 and < 0.001; chronicity: 0.934 and < 0.001; total: 0.972 and < 0.001).
Conclusion: In this interim analysis, the handheld US device with L15 scanner showed moderate-substantial agreement to detect elementary lesions of enthesitis, with the exception of calcifications. In addition, a very strong consistency was found between the devices in inflammation, chronicity and total enthesitis scores, which enable physicians to evaluate enthesitis as a whole. These interim results encourage testing the use of handheld US devices for wider use.
To cite this abstract in AMA style:
Acikgoz S, Gazel U, Shah S, Maguin M, Apurva Machhar R, Eder L, Kaeley G, Aydin S. Validation of Handheld Ultrasound Devices for Point of Care Use in Rheumatology: Interim Analysis for Enthesitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validation-of-handheld-ultrasound-devices-for-point-of-care-use-in-rheumatology-interim-analysis-for-enthesitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-handheld-ultrasound-devices-for-point-of-care-use-in-rheumatology-interim-analysis-for-enthesitis/