Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with systemic lupus erythematosus (SLE) are at increased risk of serious infections, which in turn, are associated with morbidity and mortality. The Systemic Lupus Erythematosus Registry of the Spanish Society of Rheumatology (RELESSER) group has developed and internally validated a tool for prediction of serious infections in SLE, with a recently improved version (SLE SI Score Revised or SLESIS-R)1, being an accurate and reliable instrument. SLESIS-R includes age, previous SLE-related hospitalization, previous serious infection, and glucocorticoid dose. This study aimed to validate SLESIS-R in a multi-ethnic, multi-national Latin-American (LA) SLE cohort.
Methods: GLADEL 2.0 is an observational cohort from 10 LA countries of patients ≥18 years of age who fulfilled the 1982/1997 American College of Rheumatology (ACR) and/or the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria. Patients with sufficient data at baseline and first annual visits were included. The outcome variable was any serious infection during the first year of follow up that led to hospitalization. Baseline demographics and clinical manifestations, disease activity (SLEDAI-2k), SLICC/ACR Damage Index (SDI) and treatments were examined. Logistic regression was used to examine the predictive effect of baseline variables on the development of serious infection in the first year of follow-up. Receiver operator characteristics (ROC) analysis was used to define the area under the curve (AUC) for SLESIS-R. The cut-off point with the best validity parameters (sensitivity and specificity) was identified.
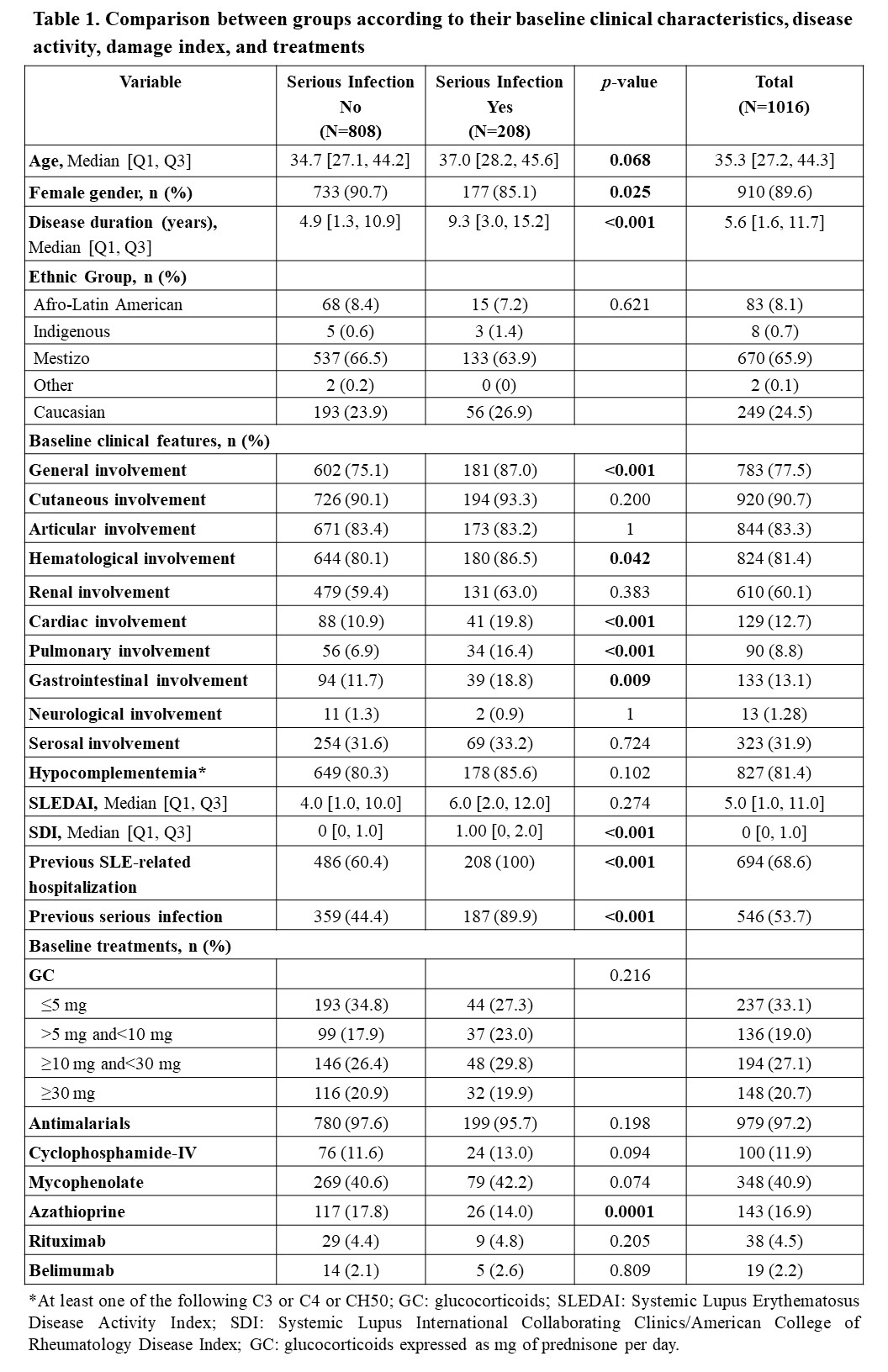
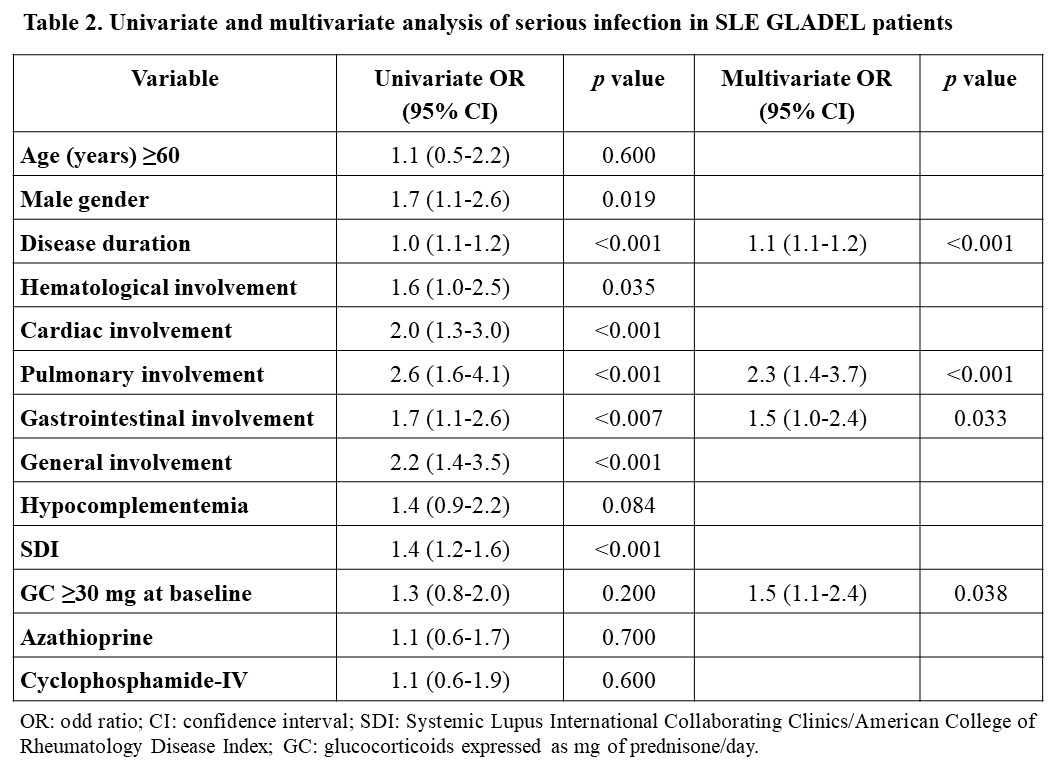
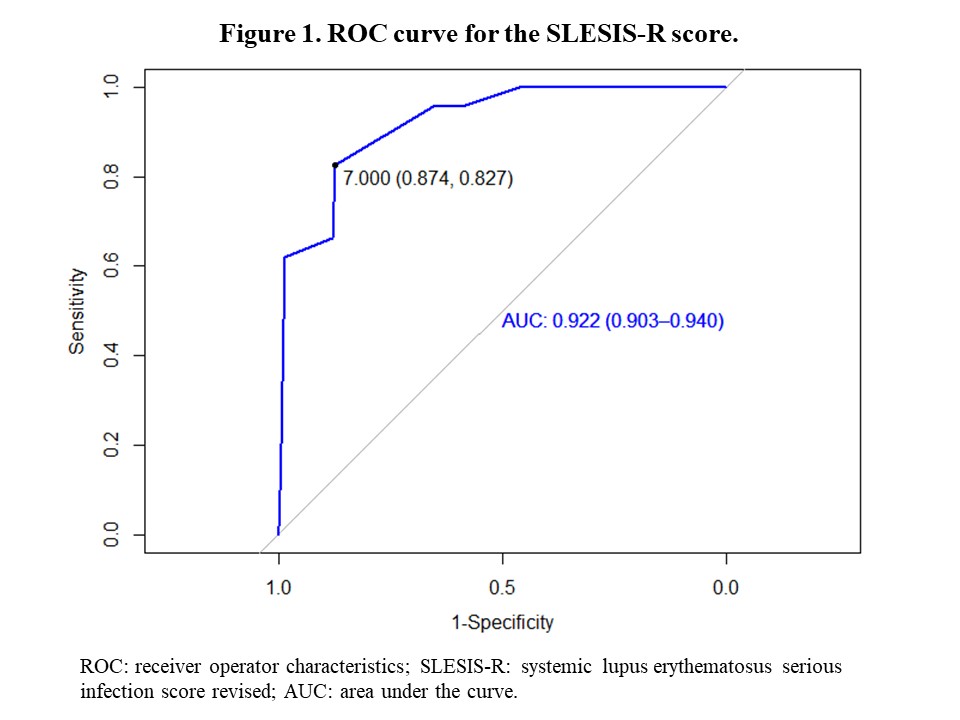
Results: Of the 1016 patients who completed one-year follow-up, 208 (20.4%) had serious infections. Patients with serious infections were older, predominantly male, and had a longer disease duration (Table 1). This group had more frequent general, cardiac, pulmonary, hematological and gastrointestinal involvement at baseline and had a higher SDI and higher proportion of previous hospitalization. Univariate and multivariate analyses (Table 2) show variables associated with serious infection: disease duration, pulmonary and gastrointestinal involvements, and baseline glucocorticoid use. The AUC for the score was 0.922 (0.903-0.940) (Figure 1). A score of 7 was chosen as the optimal cut-off point, demonstrating a sensitivity of 87% and specificity of 82%.
Conclusion: Almost a third of patients had serious infections during the first year of follow-up. The score performed well in predicting serious infections, similar to the original score.
Reference
1. Rua-Figueroa I, et al. Lupus Sci Med. 2024;11:e001096. doi:10.1136/lupus-2023-001096
To cite this abstract in AMA style:
Quintana R, Pons-Estel G, Roberts K, Palacios Santillan E, Rúa-Figueroa Í, Pego-Reigosa J, Ibañez P, Ariel Berbotto L, Constanza Bertolaccini M, Micelli M, Pisoni C, De Souza Barbosa V, de Ataíde Mariz H, Ribeiro F, Parente L, Sato E, Mimica Davet M, Aroca Martínez G, Bonilla-Abadía F, López G, Sánchez Briones R, Pérez Cristóbal M, Silveira Torre L, De La Torre I, Morales Avendaño I, Gamez-Siller P, Paats A, Calderón J, Mendoza Maldonado A, Rebella M, Silveira G, Fredy Jaramillo J, Sanchez M, Sbarigia U, Orillion A, Zazzetti F, Alarcon G, Pons-Estel B. Validation of a Score for the Prediction of Serious Infection in Patients with Systemic Lupus Erythematosus: Data from a Latin American Lupus Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/validation-of-a-score-for-the-prediction-of-serious-infection-in-patients-with-systemic-lupus-erythematosus-data-from-a-latin-american-lupus-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-score-for-the-prediction-of-serious-infection-in-patients-with-systemic-lupus-erythematosus-data-from-a-latin-american-lupus-cohort/