Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient satisfaction after total hip (THR) and total knee replacement (TKR) is a core outcome selected by the Outcomes Measurement in Rheumatology (OMERACT), yet up to 30% of THR/TKR patients are dissatisfied. Improving patient satisfaction is hindered by the lack of a validated measurement tool that can accurately measure change.
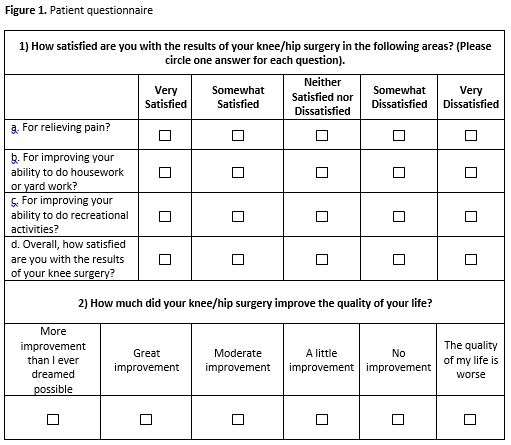
Methods: This was a retrospective analysis using prospectively collected data of 10011 patients undergoing unilateral THR and TKR between 5/ 2007 and 2/2011 in The Hospital for Special Surgery arthroplasty registry. We tested psychometric properties of a proposed satisfaction instrument composed of 4 primary questions rated on a Likert scale, scored 1-100 (Figure 1). Validity was assessed through correlation with similar constructs. We tested reliability with Cronbach’s α, and sensitivity to change was assessed through the change corresponding to minimal change or a large change in two global questions, satisfaction with TJR outcome and Quality of Life (QOL).
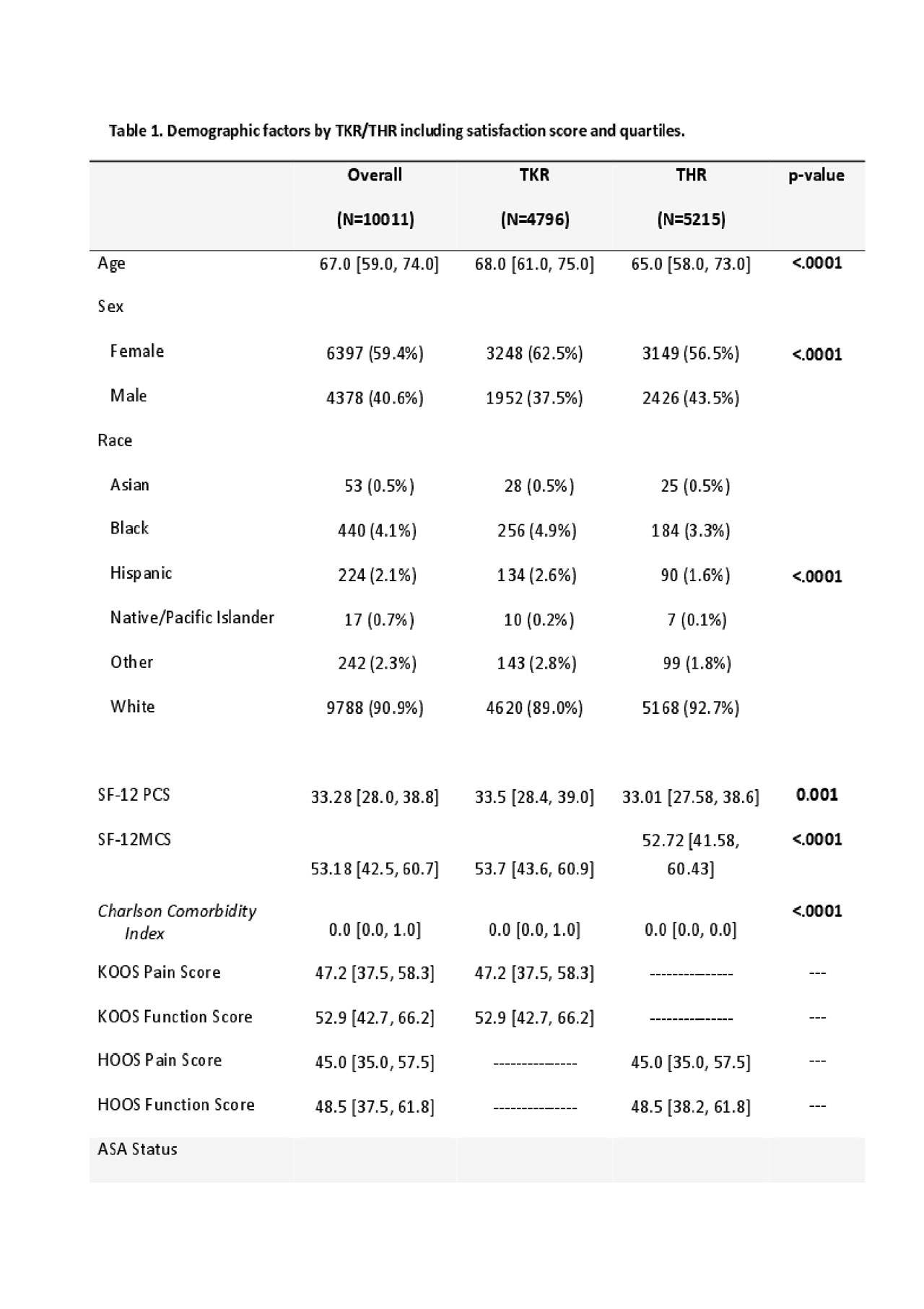
Results: We included 4,796 TKR and 5215 THR (Table 1); 90% were somewhat/very satisfied with pain relief, and almost 90% of TKR and 95% of THR were somewhat/very satisfied overall. Satisfaction correlated moderately with pain relief (TKR ρ=0.61, THR ρ=0.47) and function (TKR ρ=0.65, THR ρ=0.51) at 2 years; there was no correlation with baseline values. Reliability was demonstrated with overall Cronbach’s alpha > 9. HOOS/KOOS improvement of >30 points corresponds to a mean Satisfaction score of 93.25 (11.53) after THR and 90.37 (SD 13.76) after TKR. When HOOS/KOOS pain and function were divided in to quartiles, increasing relief of pain and functional improvement increased the strength of the association of 2 core domains with satisfaction.
Conclusion: Satisfaction with THR/TKR can be measured with 4 primary item questions. Moderate correlations with pain and function confirm that satisfaction is a separate construct. This satisfaction measure can be included in a TJR core measurement set for TJR trials.
To cite this abstract in AMA style:
Goodman S, Mehta B, Kahlenberg C, Finik J, Figgie M, Parks M, Padgett D, Antao V, Yates A, Springer B, Lyman S, Singh J. Validation of a Satisfaction Measure for Use in Total Joint Replacement Clinical Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/validation-of-a-satisfaction-measure-for-use-in-total-joint-replacement-clinical-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/validation-of-a-satisfaction-measure-for-use-in-total-joint-replacement-clinical-trials/