Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologic Disease-Modifying agents (bDMARDs) including TNF inhibitors are increasingly used during pregnancy over the last few decades for a variety of autoimmune conditions. Nevertheless, limited data is available regarding their safety through pregnancy, particularly in the third trimester. Previous reports of discriminated BCG and severe neonatal neutropenia has made many practitioners defer live-attenuated vaccines from the routine schedule. The international guidelines still recommend holding most TNF inhibitors for several half-lives before delivery, due to the significant levels found in the neonates. Many mothers are advised to adjust the immunization schedule of their infants accordingly.
Recent accumulating data from IBD in pregnancy started to support the safety of infliximab use throughout pregnancy. Due to the paucity of data in this regard, we have planned this study.
Methods: We surveyed the electronic records of two registries from the IBD and Rheumatology clinics at the largest tertiary hospital in Qatar. We included all women who completed their pregnancies on biologics (minimum of 3 months of biologic use during pregnancy). Cases of abortion, IUFD, and neonatal death were excluded. We have analyzed the clinical data of infants who were 6 months of age or older. Data were recorded in a pre-specified datasheet. Then we ran a descriptive analysis using SPSS software.
Results: Since 2017, we identified 30 completed pregnancies on TNF blockers. Out of these, twenty-five infants were more than 6 months of age.
Mean maternal age at pregnancy was 29.5 years (22-42)
Underlying autoimmune diseases: IBD (13), RA (7), PSA(2), SPA (3).
TNF inhibitors used: Infliximab(7), Adalimumab(5), Etanercept(2), and Certolizumab (10).
The mean duration of TNF inhibitor use during pregnancy was 6.8 months (5-9 months).
TNF inhibitors were taken throughout pregnancy in 15 cases: certolizumab (6), Infliximab (4), and adalimumab (2).
In 2 pregnancies, Etanercept was taken for 7 months followed by Certolizumab until birth.
Mean gestational age at birth was 36.8 weeks (34-41).
Prematurity was recorded in 7 cases (34-37 weeks) and LBW in 1 case.
Two neonates required NICU admission for ARDS.
Five cases of neonatal jaundice were identified. No congenital anomalies reported.
Immunization Data:
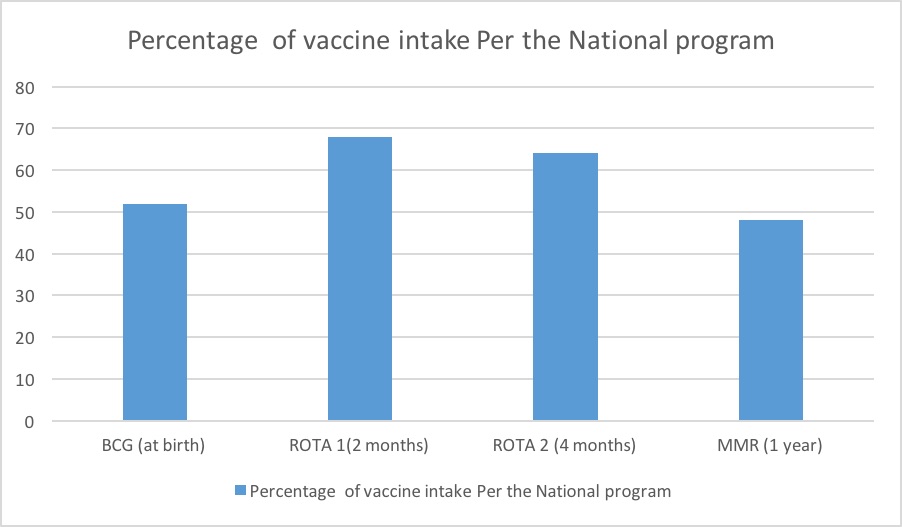
Live attenuated vaccines: Thirteen infants (52%) received BCG vaccine at birth, 17(68%) received ROTA vaccine at 2 months, 16 (64%) received ROTA booster at 4 months and 12 (46%) infants received MMR at 1 year.
No complications related to the vaccines were recorded.
Infections during Infancy:
Mean number of infection encounters during infancy was 2 encounters (0-5).
Infections reported were: URTI, bronchiolitis and otitis media. One infant got COVID19 mild infection.
Mean number of encounters that required antibiotics was 10 in 9 infants.
Conclusion: We have identified high rate of infants who received live-attenuated vaccines per the national immunization program without complications. This observation was also associated with high rate of maternal TNF inhibitor use throughout pregnancy. No alarming concern about repeated infections reported in this study.
To cite this abstract in AMA style:
Satti E, Hadwan N, Al Emadi S. Vaccinations of Infants Born to Mothers on TNF Inhibitors: Safe Administration of Live-vaccines Given Per the National Immunization Program [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/vaccinations-of-infants-born-to-mothers-on-tnf-inhibitors-safe-administration-of-live-vaccines-given-per-the-national-immunization-program/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vaccinations-of-infants-born-to-mothers-on-tnf-inhibitors-safe-administration-of-live-vaccines-given-per-the-national-immunization-program/