Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Treatment of autoinflammatory periodic syndromes with the interleukin-1β inhibitor canakinumab (CAN) has been shown to be safe and effective in clinical trials and in practice. Patients are recommended to be vaccinated against common infections (including influenza, covid-19, pneumococcus) while on therapy, as is the general population. It is known from the literature that severe local and systemic inflammatory reactions can occur frequently in patients with immunosuppressive therapy, especially after pneumococcal vaccination. Therefore, in the present study, in addition to general safety parameters, the safety of the recommended vaccinations in patients with CAPS, FMF, HIDS/MKD and TRAPS under CAN therapy was investigated in clinical practice.
Methods: RELIANCE is a prospective, non-interventional observational study in Germany enrolling pediatric (age ≥2 years) and adult patients with a clinically confirmed diagnosis of autoinflammatory periodic syndrome who routinely receive CAN. Efficacy and safety parameters were recorded at baseline and assessed at 6-months intervals.
Results: The interim analysis includes data from N=199 patients with autoinflammatory diseases enrolled in the RELIANCE registry between October 2017 and December 2021. The mean age of the overall cohort is 24.4 years (2-79 years; N=104 female patients [53%]) and the median duration of CAN treatment before study entry was 2 years (0-12 years).
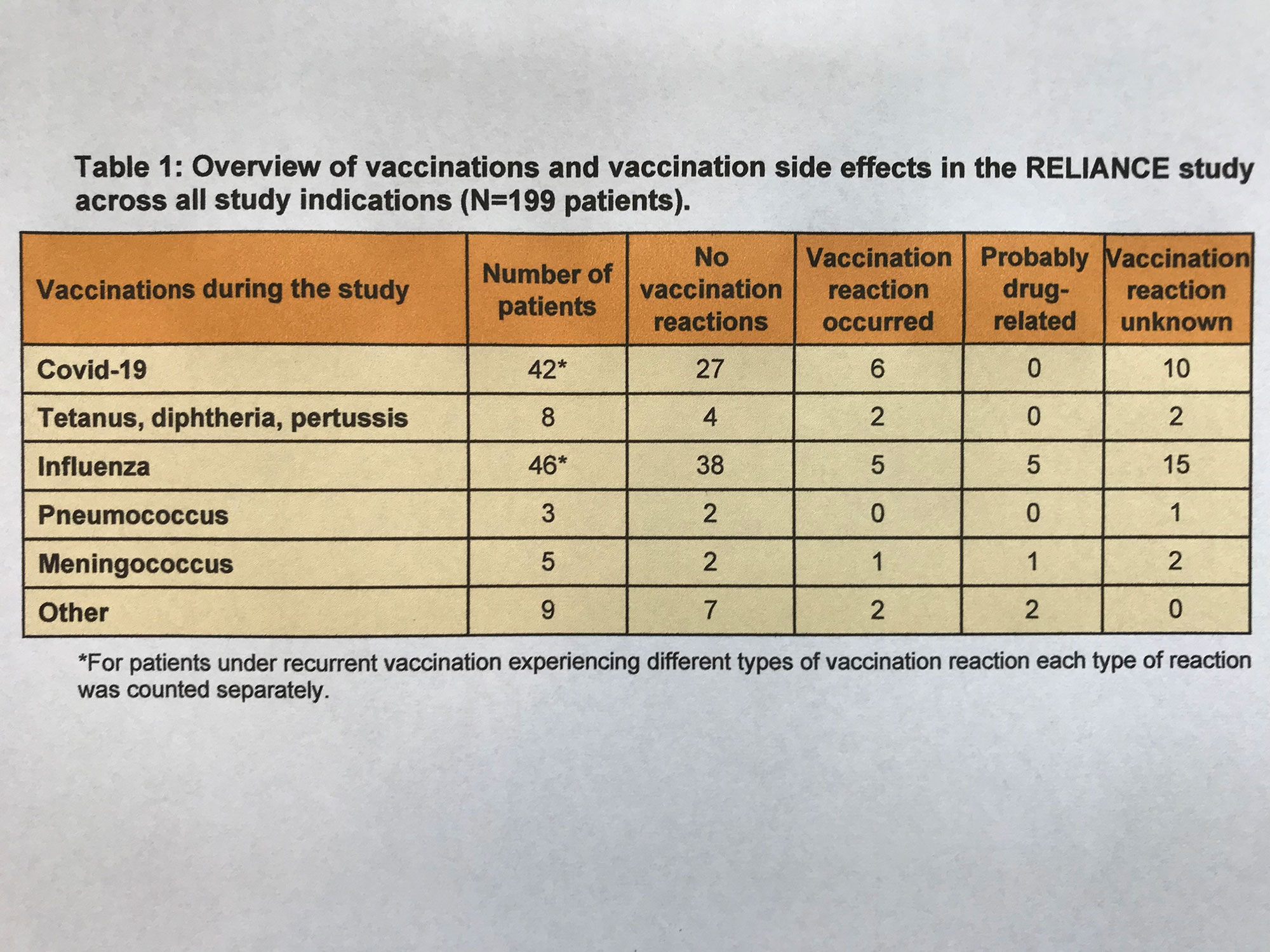
During the study, N=87 patients received a total of 130 vaccinations. Vaccination reactions were reported for N=16 patients, and N=8 patients were classified as suspected adverse drug reactions (Table 1). In no case was the vaccination reaction classified as severe.
Covid-19 vaccination was given to N=42 patients (N=6 Comirnaty, N=1 Spikevax, N=36 not reported; 1 patient received 2 different vaccines). Of these, vaccination reactions were reported for N=6 patients, which were not considered drug-related or classified as severe.
Conclusion: The interim data from the RELIANCE study confirm the safety of long-term treatment with canakinumab in the entire study population. Vaccination while on CAN therapy also did not reveal any new safety signals beyond known vaccine side effects.
To cite this abstract in AMA style:
Kuemmerle-Deschner J, Henes J, Kortus-Goetze B, Kallinich T, Oommen P, Rech J, Krickau T, Weller-Heinemann F, Horneff G, Janda A, Foeldvari I, Schuetz C, Dressler F, Borte M, Hufnagel M, Meier F, Fiene M, Weber-Arden J, Blank N. Vaccination in Patients with Autoinflammatory Periodic Syndromes Under Canakinumab – Safety Data Interim Analysis of the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/vaccination-in-patients-with-autoinflammatory-periodic-syndromes-under-canakinumab-safety-data-interim-analysis-of-the-reliance-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vaccination-in-patients-with-autoinflammatory-periodic-syndromes-under-canakinumab-safety-data-interim-analysis-of-the-reliance-registry/