Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Some concerns have been raised with the use of vaccines against SARS-CoV-2 in patients with Systemic Autoimmune Diseases (SAD) including the risk of serious adverse events. Our objetive was to describe the safety of vaccines against SARS-CoV-2 in people with SAD.
Methods: We used data from the European Alliance of Associations for Rheumatology (EULAR) Coronavirus Vaccine (COVAX) physician-reported registry (EULAR-COVAX). EULAR-COVAX recently reported global results in inflammatory and non-inflammatory rheumatic and musculoskeletal diseases (RMD) (1). We included patients with SAD reported to the registry from February 2021 to 3 March 2022 including patients with large, medium and small vessel vasculitis, ANCA-associated vasculitis, systemic lupus erythematosus (SLE), primary antiphospholipid syndrome (APS), Sjögren syndrome (SS), systemic sclerosis (SSc), inflammatory myositis, mixed connective tissue disease (MCTD) and undifferentiated connective tissue disease (UCTD). Patients with giant cell arteritis (GCA) and polymyalgia rheumatica (PMR) were grouped. Data on demographics, vaccination, RMD diagnosis, disease activity, immunomodulatory/ immunosuppressive treatments, early adverse events (reactogenicity) and adverse events of special interest were collected. Data were analysed descriptively.
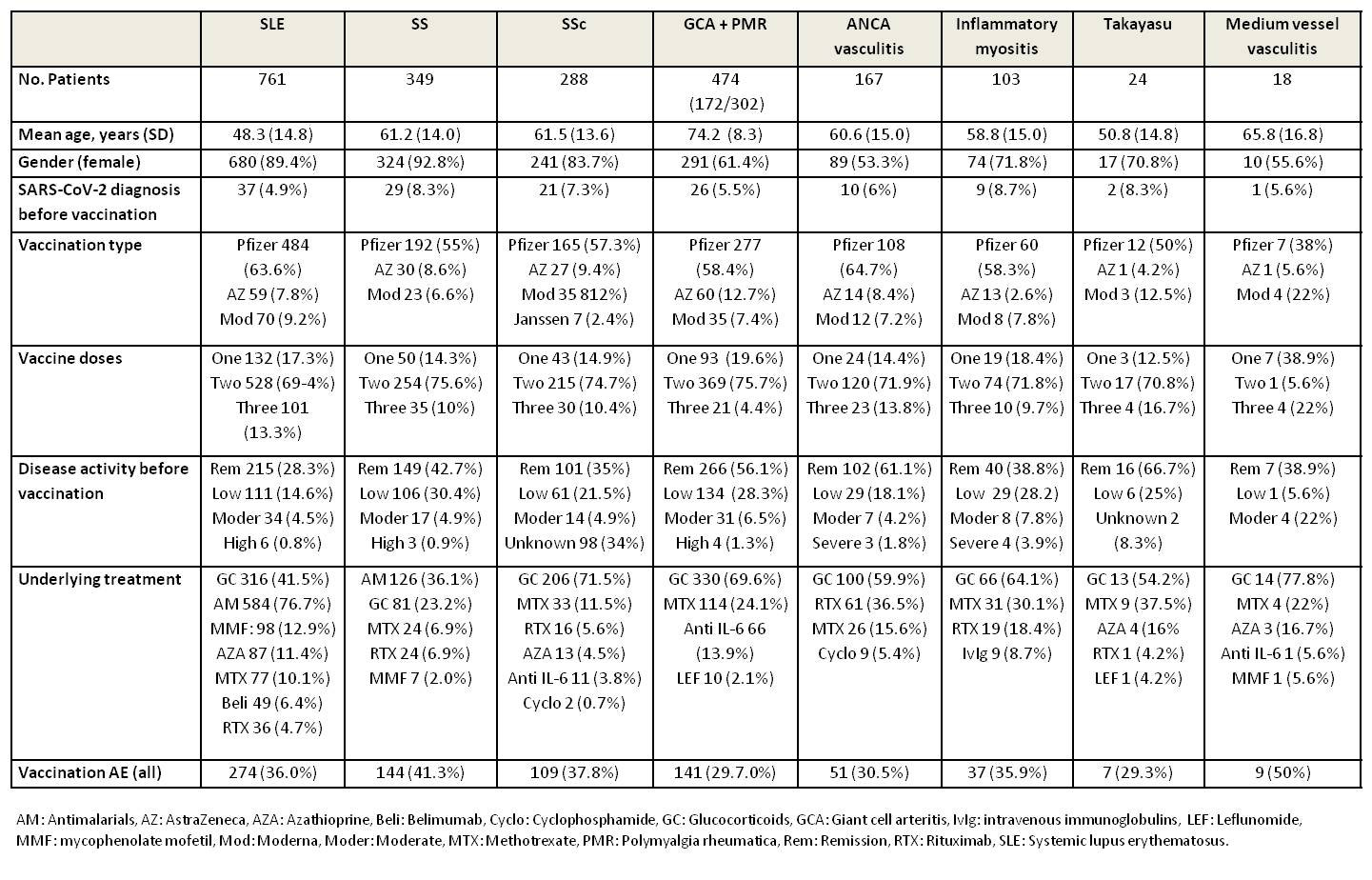
Results: A total of 2570 (24.3%) out of 10569 patients included in the registry had a diagnosis of SAD. The study included patients from 31 countries, mostly female (76.7 %), with a mean age of 57.8 (SD 16.6) years. A total of 164 (6.4%) patients had previous SARS-CoV2 infection before vaccination. The main diagnoses included SLE (n=761), GCA plus PMR (n=474), SS (n=349), SSc (n=288), ANCA-associated vasculitis (n=167), inflammatory myositis (n=103), UCTD (n=86), MCTD (n=53), primary APS (n=40), Takayasu (n=24), medium vessel vasculitis (n=18) and other vasculitis (n=11). Clinical characteristics of patients with more common SAD are shown in Table 1.
A total of 1618 (62.9%) early adverse events were reported. Pain at the site of injection in 480 (18.6%) patients, fatigue in 285 (11.0%), fever in 205 (7.9%) generalized muscle pain in 176 (6.8%), headache in 159 (6.1%), among others. Adverse events of special interest were reported in 53 (1.9%) patients (46 mild or moderate and 7 severe).
Conclusion: In this international physician-reported SARS-CoV-2 vaccination registry, patients with SAD had a favourable outcome with very good vaccine tolerance and safety. The frequency of early side effects (reactogenicity) was similar to the general population. Only a very small proportion of patients (< 2%) had adverse events of special interest and most of them were mild or moderate.
To cite this abstract in AMA style:
Gomez-Puerta J, Martins Fernandes A, Sarmiento-Monroy J, Lawson-Tovey S, Hyrich K, Gossec L, Carmona L, Strangfeld A, Mateus E, Rodrigues A, Hachulla E, Mosca M, Durez P, Raffeiner B, Roux N, Eric V, Brocq O, Zepa J, Strakova E, Bulina I, Mlynarikova V, Šteňová E, Soubrier M, Mariette X, Machado P. Vaccination Against SARS-CoV2 in Patients with Systemic Autoimmune Diseases: A Safety Report from EULAR COVAX Registry [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/vaccination-against-sars-cov2-in-patients-with-systemic-autoimmune-diseases-a-safety-report-from-eular-covax-registry/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vaccination-against-sars-cov2-in-patients-with-systemic-autoimmune-diseases-a-safety-report-from-eular-covax-registry/