Session Information
Date: Saturday, November 6, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster I (0387–0413)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: COVID-19 vaccination is recommended for individuals with rheumatic diseases, yet data are limited regarding vaccine safety in this population, particularly among those with rare autoimmune conditions such as systemic sclerosis (SSc; scleroderma). The purpose of this cross-sectional, observational study was to evaluate the self-reported experiences of individuals with SSc who have received at least one dose of a COVID-19 vaccine.
Methods: Participants were adults with physician-verified SSc enrolled in the Scleroderma Patient-centered Intervention Network (SPIN) Cohort. SPIN includes 47 international centers and has approximately 1600 active participants. From April 9 to May 15, 2021, participants from the SPIN Cohort were invited by email and popups during regular SPIN Cohort assessments to participate in a COVID-19 vaccine survey, which was conducted in English and French. The survey inquired about whether participants had received the COVID-19 vaccine and their experience with vaccination. Frequencies and means (standard deviation (SD)) are reported. Characteristics are compared between those with and without self-reported adverse reactions using chi-square or t-test. Logistic regression was used to compare likelihood of self-reported adverse reactions by vaccine type.
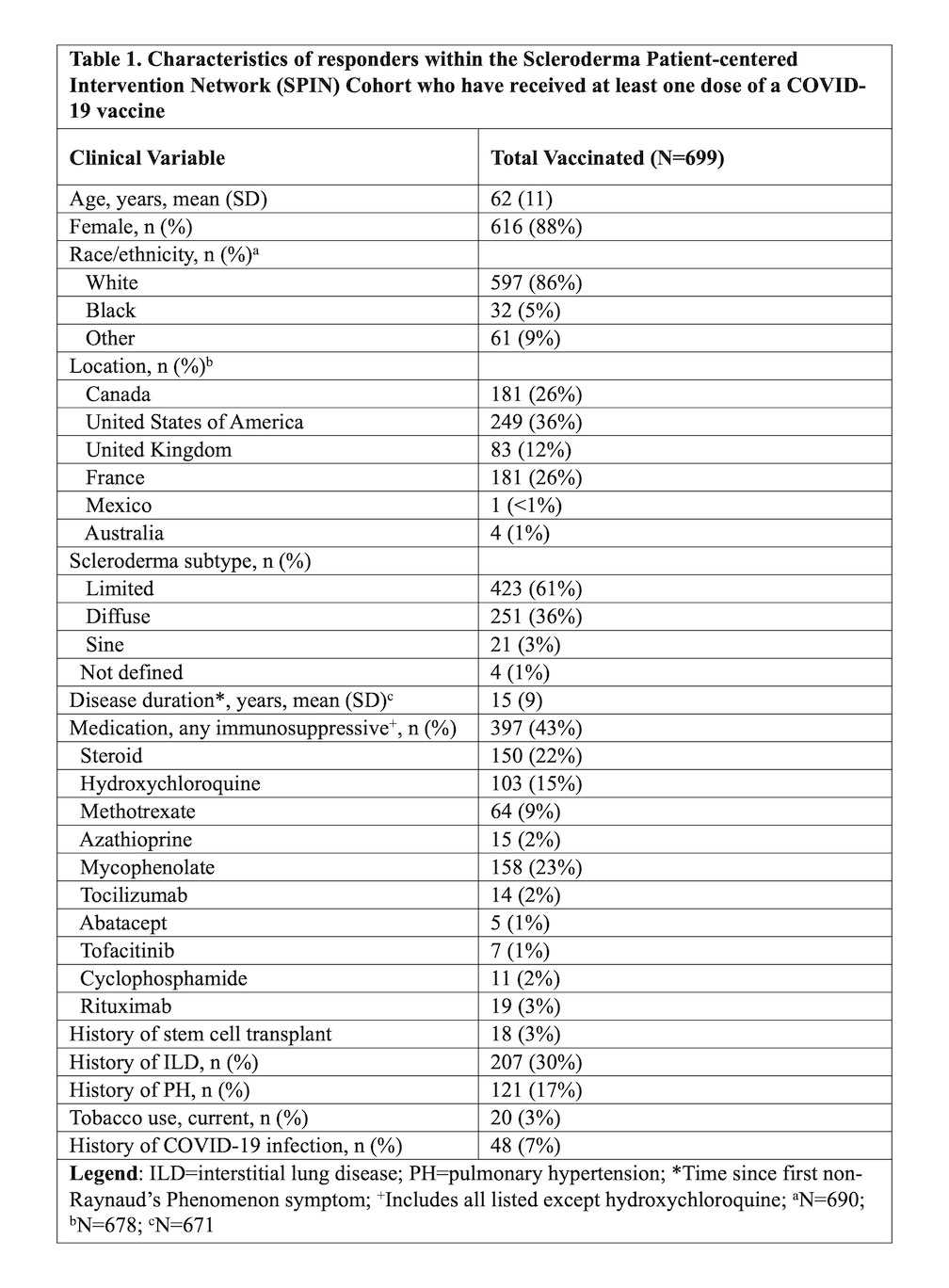
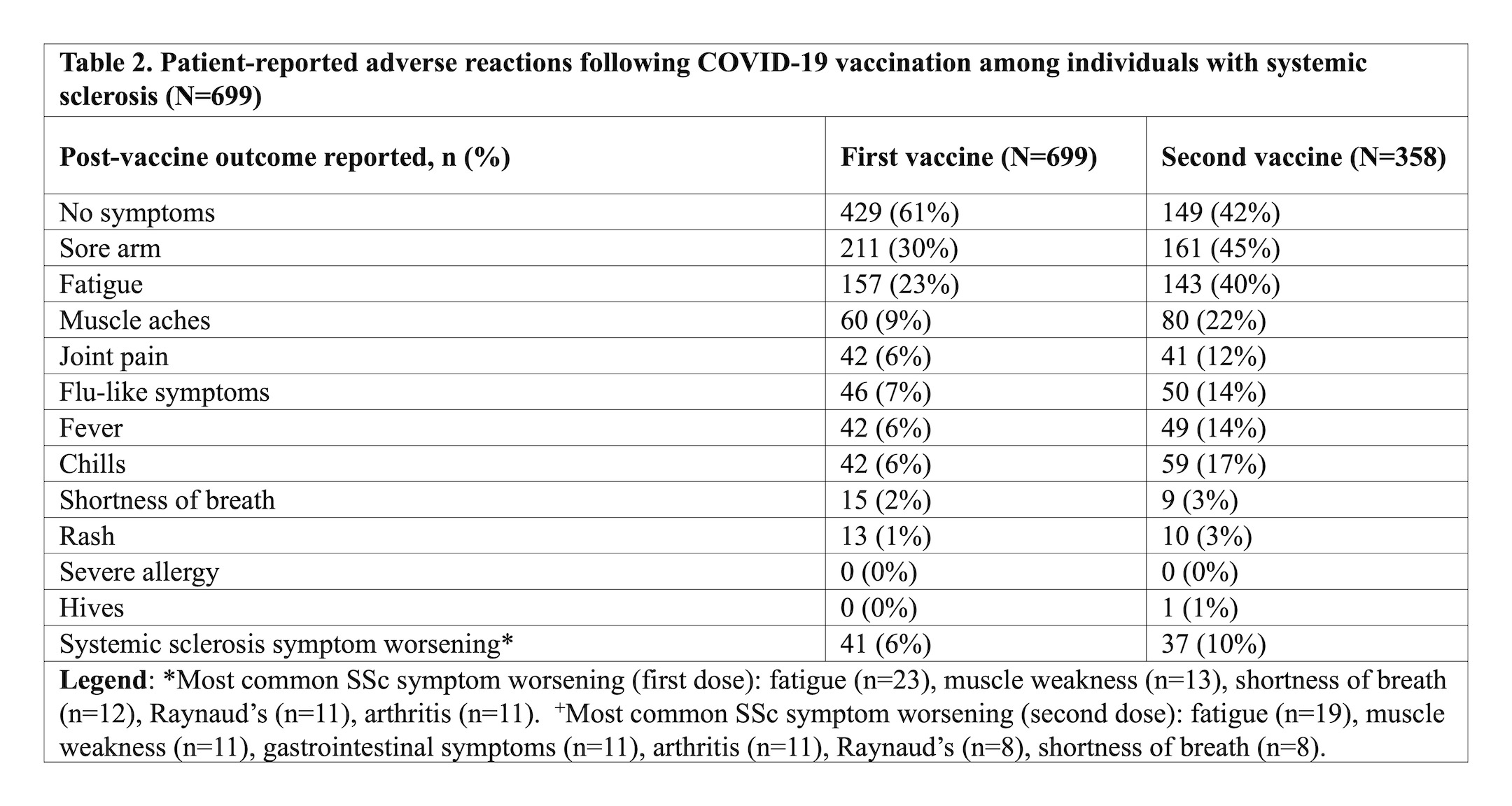
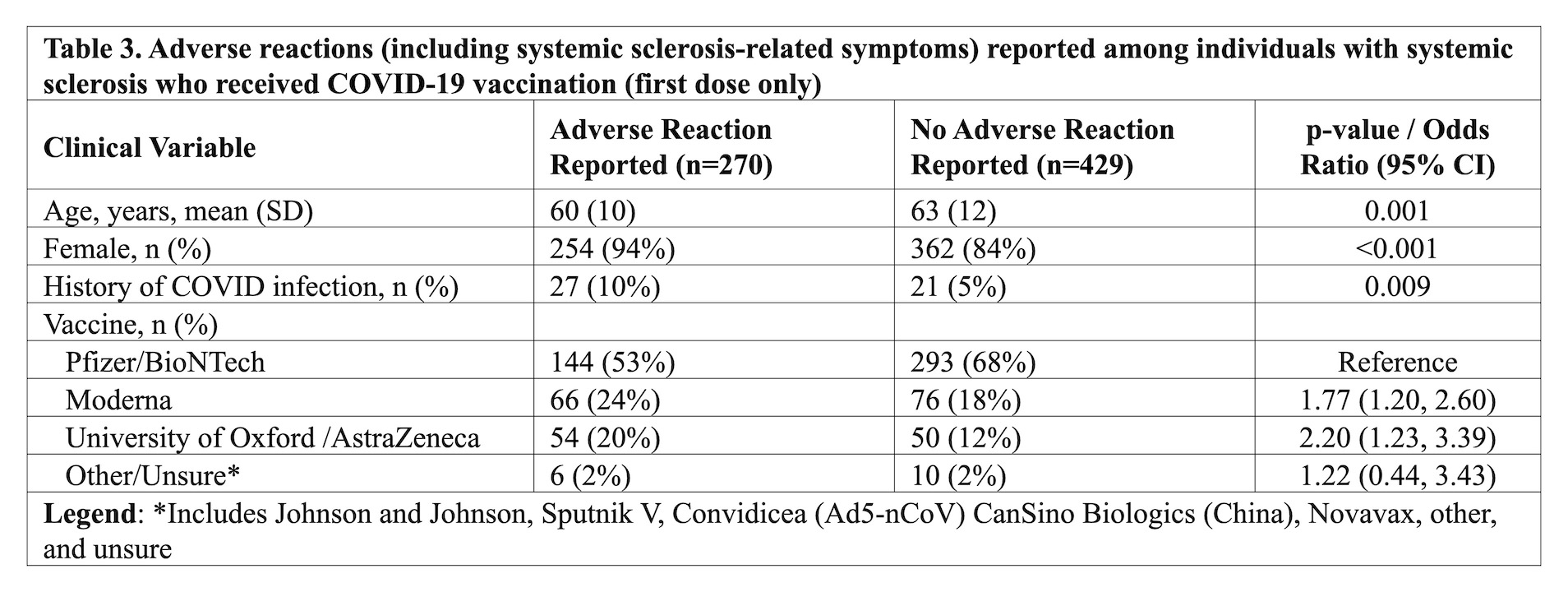
Results: Among 932 responders, 699 (75%) had received at least one dose of a COVID-19 vaccine, and 358 (51%) had also received a second dose. The majority of participants received the Pfizer vaccine (63%). Demographic data are presented in Table 1. Most responders did not make changes to their SSc medications for the first (94%) or second (92%) vaccine dose. Adverse reactions were self-reported in 270 individuals (39%) after the first vaccine dose and 209 individuals (58%) after the second vaccine dose (Table 2). The three most common symptoms after the first and second vaccine dose were sore arm (30% and 45%, respectively), fatigue (23% and 40%, respectively), and muscle aches (9% and 22%, respectively). No serious allergic reactions were reported. Self-reported flares of SSc symptoms were rare after the first (6%) and second vaccine dose (10%), and most common symptoms were fatigue and muscle weakness (Table 2). Those with (vs. without) self-reported adverse reactions (any) to the COVID-19 vaccine were younger (60 vs. 63, p=0.001), more often female (94% vs. 84%, p< 0.001), and more often had a prior history of COVID-19 (10% vs. 5%, p=0.009) (Table 3). Compared to those who received the Pfizer vaccine, the odds of a self-reported adverse reaction were higher for those who received Moderna (OR 1.77, 95% CI 1.20, 2.60) or AstraZeneca (OR 2.20, 95% CI 1.43, 3.39) vaccines.
Conclusion: The rate and types of self-reported adverse reactions to the COVID-19 vaccine among individuals with SSc were similar to those observed in the general population. Few individuals altered their medication regimen for the vaccine, and self-reported worsening of SSc symptoms was rare.
To cite this abstract in AMA style:
Showalter K, Gordon J, Wu Y, Kwakkenbos L, Carrier M, Henry R, Østbø N, Nordlund J, Bourgeault A, Canedo Ayala M, Discepola M, Carboni Jiménez A, Denton C, Mouthon L, Thombs B, Spiera R. Vaccination Against COVID-19: Self-Reported Experiences of Patients with Systemic Sclerosis in the Scleroderma Patient-centered Intervention Network (SPIN) Cohort [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/vaccination-against-covid-19-self-reported-experiences-of-patients-with-systemic-sclerosis-in-the-scleroderma-patient-centered-intervention-network-spin-cohort/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/vaccination-against-covid-19-self-reported-experiences-of-patients-with-systemic-sclerosis-in-the-scleroderma-patient-centered-intervention-network-spin-cohort/