Session Information
Date: Monday, November 14, 2016
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment III: Novel and Current Therapies
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Preliminary validation of a Lupus Low Disease Activity State (LLDAS) definition has demonstrated that LLDAS attainment is associated with reduced damage accrual in patients (pts) with SLE.1 However, it has not been evaluated in randomized controlled trials (RCTs). We present a post-hoc analysis evaluating LLDAS in the MUSE trial of anifrolumab in pts with moderate to severe SLE.2
Methods:
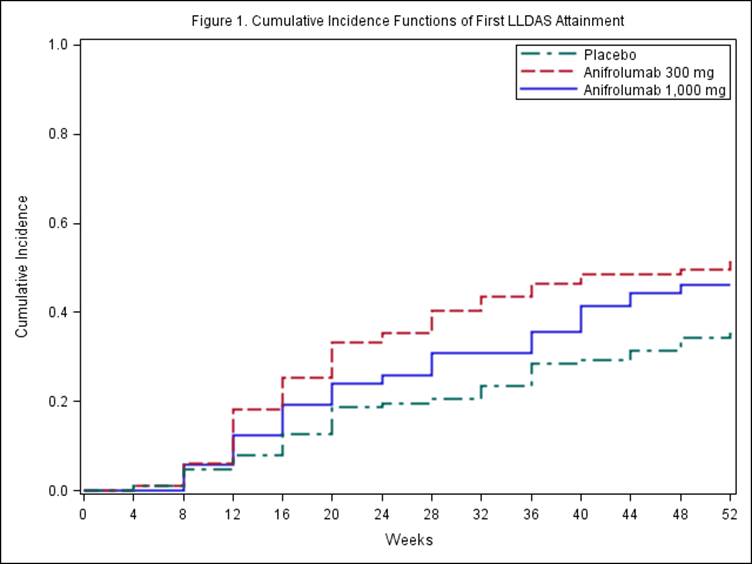
LLDAS requires all of the following: SLEDAI–2KResults:
During the 52-week study period of MUSE, pts received intravenous placebo (n=102), anifrolumab 300 mg (n=99), or anifrolumab 1,000 mg (n=104), in addition to standard of care, every 4 weeks for 48 weeks. LLDAS criteria were met, at least once, by 35%, 52%, and 46% of pts, respectively (odds ratio [OR] vs. placebo; 300 mg: 1.97, 90% CI 1.19, 3.25;Conclusion:
In the MUSE study, LLDAS correlated with clinically relevant definitions of treatment response and discriminated responders from non-responders. Treatment with anifrolumab 300 mg was associated with up to 3.6-fold increase in odds of LLDAS attainment. LLDAS should be considered as an outcome measure in SLE RCTs.To cite this abstract in AMA style:
Morand E, Berglind A, Sheytanova T, Tummala R, Illei G. Utility of the Lupus Low Disease Activity State Definition in Discriminating Responders in the Phase IIb Muse Trial of Anifrolumab in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/utility-of-the-lupus-low-disease-activity-state-definition-in-discriminating-responders-in-the-phase-iib-muse-trial-of-anifrolumab-in-systemic-lupus-erythematosus/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-the-lupus-low-disease-activity-state-definition-in-discriminating-responders-in-the-phase-iib-muse-trial-of-anifrolumab-in-systemic-lupus-erythematosus/