Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient Reported Outcomes (PROs) can provide important data about the impact of a disease on an individual and/or the quality of the response to medication. However, in most circumstances, PRO information is collected only intermittently and usually at the point of care or treatment. The development of mobile technology to collect PRO data electronically (ePRO) provided the opportunity to acquire this information more frequently, in real time, and in the person’s normal environment. The objective of the research was to develop a smart phone application (app) and test its utility to collect ePRO information in people with systemic lupus erythematosus (SLE).
Methods: A smart phone app was developed that collects data from a number of PRO instruments, including FACIT-F (fatigue), SF-36 (health-related quality of life) and patient global assessment (PtGA). People living with SLE were involved in the initial development and evaluation of the acceptability of the app. To test to utility of this app, a multi-center clinical study (VALUE, NCT03142711) was carried out in collaboration with people with SLE, in whom ePRO information was collected with the app daily (PtGA) or weekly (FACIT-F and SF-36) in the person’s environment and also with the app and a paper form monthly at the clinical site. Demographic information, compliance and intra-class correlation coefficients between information collected with the app and using a paper form at the clinical site were assessed.
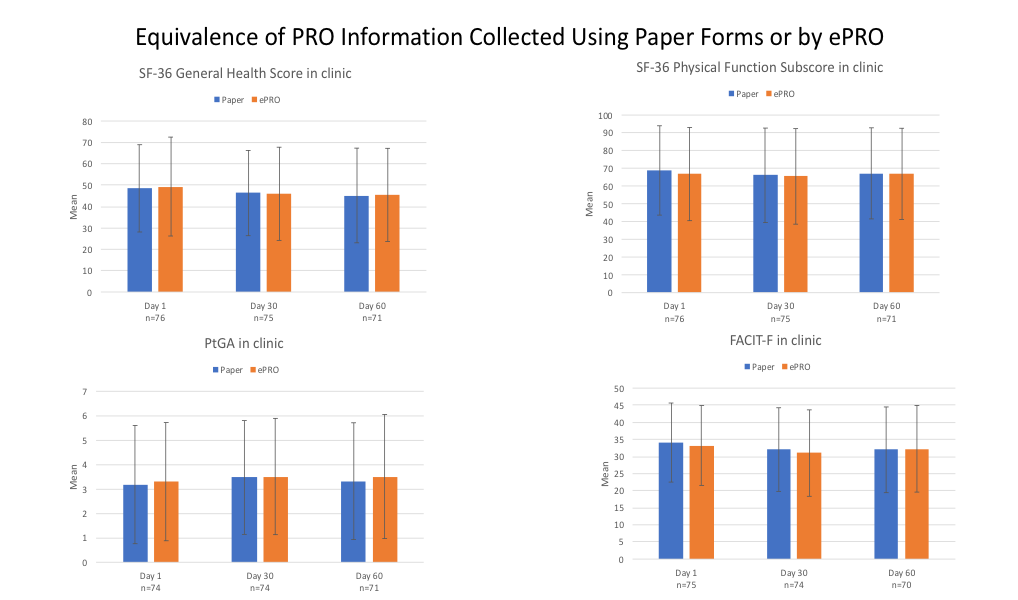
Results: Of the 80 people enrolled in this study, 91.3% were women; 57.5%, 16.3% and 17.5% identified themselves as of European, African or Asian ancestry, respectively. The mean age was 42.6 years and the mean duration of education was 15.6 years. Overall compliance with completing the ePRO instruments with the app at the scheduled time was 88%. To determine the consistency of information collected with the app, PRO instruments were completed on three occasions: a) in standard fashion using a paper form and b) using the app, separated by an interruption at the clinical site. The mean (SD) PtGA, FACIT-F and SF-36 Physical Functioning and General Health scores at baseline, month 1 and month 2 using the app and paper forms were similar (Figure 1). The Intraclass Correlation Coefficient (ICC) and 90%CI were 0.97 (0.96-0.98), 0.96 (0.94-0.07) and 0.95(0.93-0.97) at the three time points, respectively for the PtGA; 0.94(0.91-0.96), 0.96(0.95-0.98) and 0.96(0.94-0.97), respectively for the FACIT-F instrument; and , 0.96(0.94-0.97), 0.94(0.91-0.96) and 0.96 (0.94-0.97) for the 3 time points, respectively for the SF-36 Physical Functioning score.
Conclusion: Compliance with completion of ePROs using a mobile app was high and the content collected with the app conformed with that collected using a paper form. Since compliance with the use of a mobile app to collect ePRO information and the consistency of the information obtained compared to that obtained using a paper form were high, the app affords the potential opportunity to acquire frequent and highly reliable information about the impact of disease and response to medication in individuals with SLE.
To cite this abstract in AMA style:
Williams B, Muckian B, Dykas C, Peschken C, Furie R, Massarotti E, sikirica V, Gilbert S, Hodge M, Lipsky P. Utility of a Mobile Phone Based Application to Collect Patient-Reported Outcome Information from People Living with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/utility-of-a-mobile-phone-based-application-to-collect-patient-reported-outcome-information-from-people-living-with-systemic-lupus-erythematosus/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-a-mobile-phone-based-application-to-collect-patient-reported-outcome-information-from-people-living-with-systemic-lupus-erythematosus/