Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: In the PsABio study (NCT02627768), real-world data from patients with PsA showed comparable clinical results after treatment with ustekinumab (UST, an anti-p40 IL-12/23 inhibitor) versus TNF inhibitors (TNFi).1,2 Patients of all ages may acquire psoriatic arthritis (PsA) and may require prolonged treatment. In general, treatment persistence is a function of effectiveness, safety, convenience, and treatment satisfaction. Elderly patients may be at increased risk of treatment interruption due to adverse events (AEs), increased comorbidities, and/or polypharmacy. Here we report PsABio results in younger versus older patients treated with UST.
Methods: PsABio is a multinational, prospective, observational study in patients with PsA prescribed either UST or TNFi (as 1st, 2nd or 3rd line treatment) at the discretion of the treating rheumatologist. This post-hoc analysis compared the effectiveness and safety in UST-treated patients by age subgroup (< 60 vs ≥60 years old). Drug persistence was compared by means of Kaplan-Meier plots and the log-rank test. Cox-regression analysis was used to compare the risk of stopping treatment before 15 months of follow-up between the age subgroups.
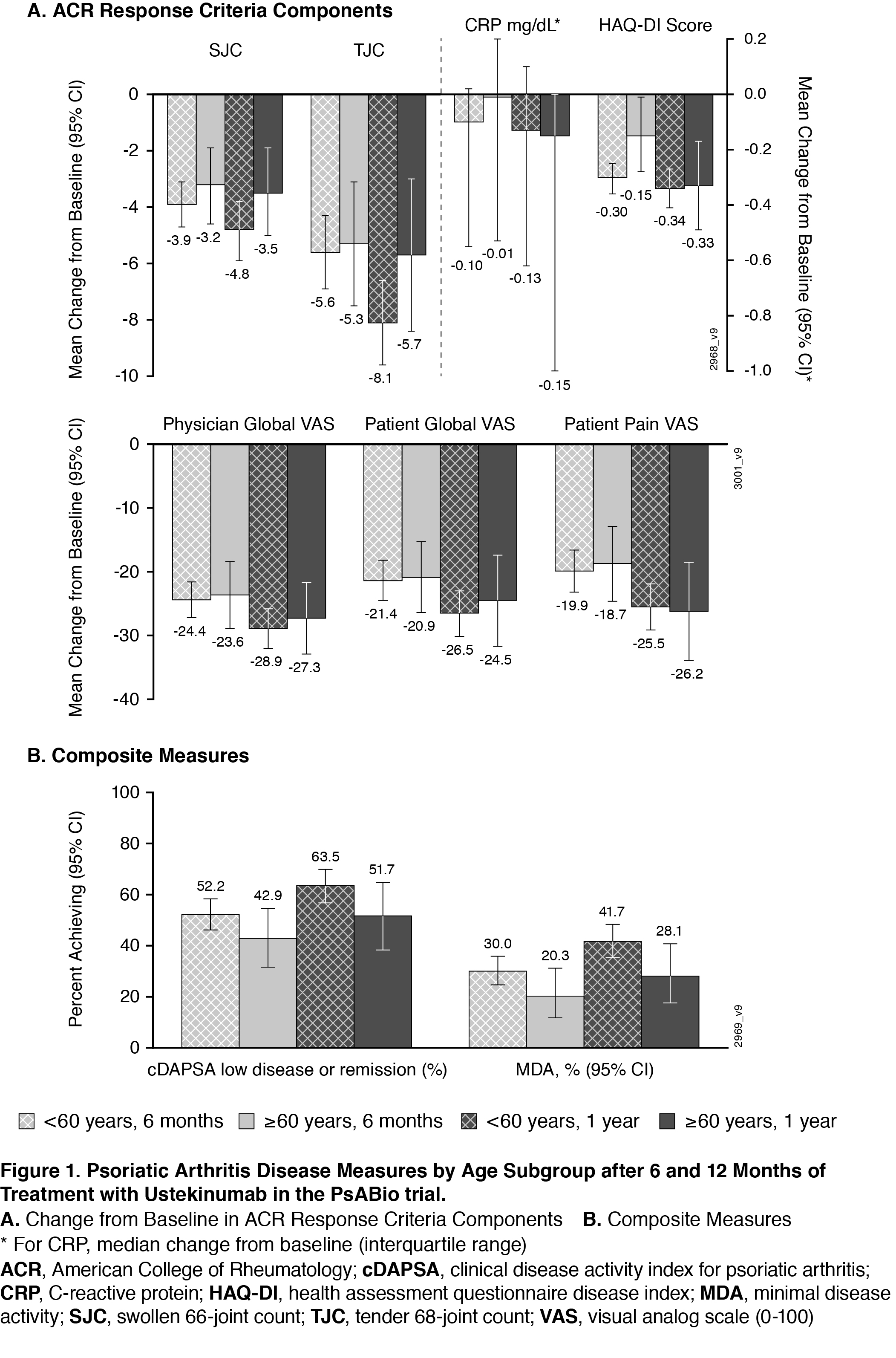
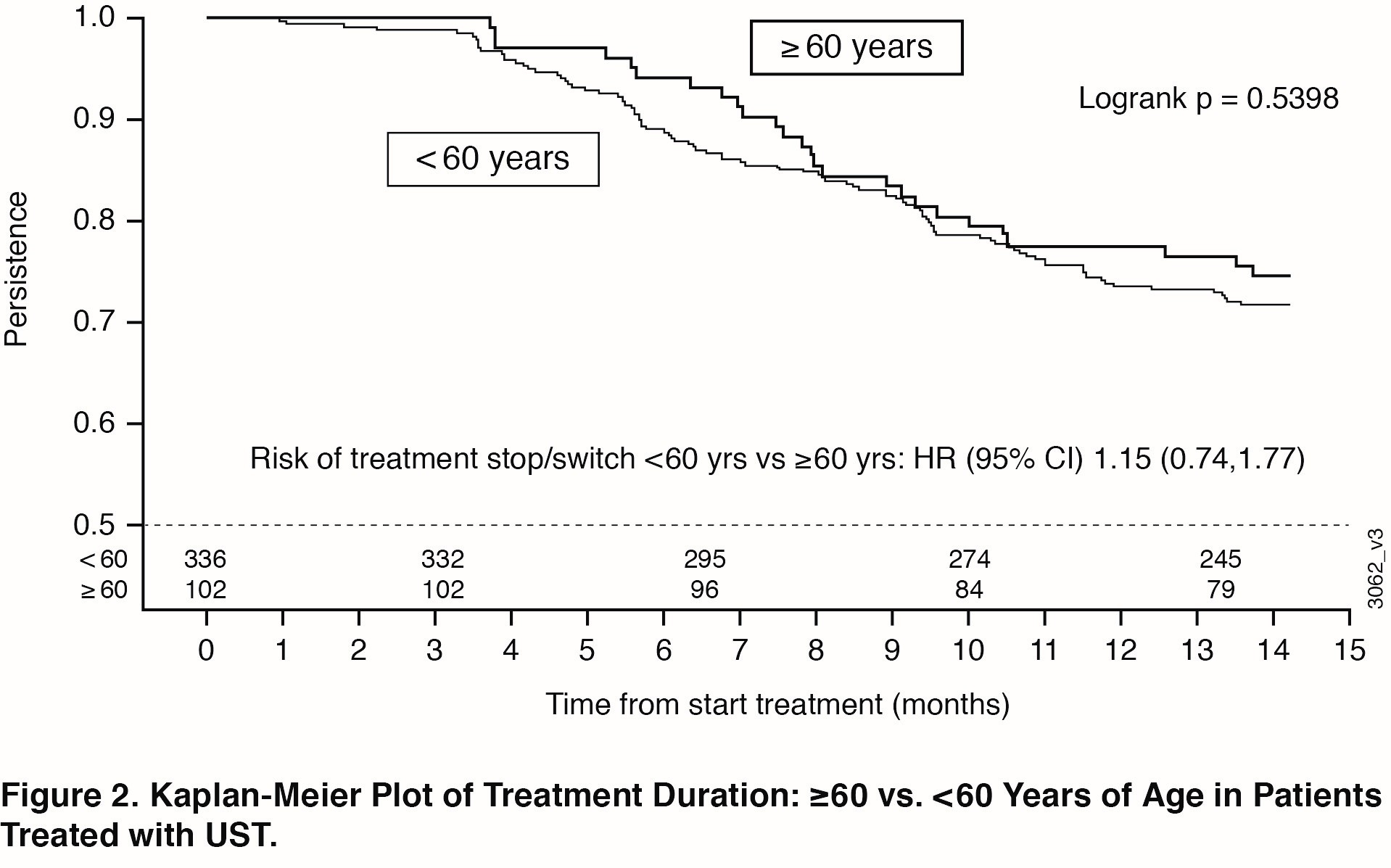
Results: 458 of 930 patients in PsABio received UST (77.1% were < 60 years of age; 22.9% were ≥60). Baseline characteristics (Table) were comparable across the older vs younger subgroups, except that older patients had more cardiovascular disease and trended towards greater PsA disease duration. Effectiveness after treatment with UST for 6 months and 1 year was generally comparable across age subgroups. The similarities included components of the ACR response criteria (Swollen 66-joint Count, Tender 68-joint Count, C-Reactive Protein, Health Assessment Questionnaire-Disease Index [HAQ-DI], Physician’s Global Assessment, Patient’s Global Assessment, and Patient’s Pain Assessment) (Figure 1A) and composite measures (clinical Disease Activity Index for Psoriatic Arthritis and Minimal Disease Activity) (Figure 1B). Numerically fewer older patients reached the goals measured in the composite endpoints. Incidence of AEs, but not withdrawal due to an AE, was somewhat higher in the older patient subgroup (data not shown). Observed treatment persistence did not differ between older and younger patients (Log Rank p value=0.54), confirmed by Cox-regression analysis showing similar risk for stop/switch within the first year (Figure 2).
Conclusion: In a real-world setting, no clinically meaningful differences were observed in UST effectiveness and safety in PsA patients who were < 60 compared with ≥60 years of age. These results were generally maintained from 6 months to 1 year of treatment. Additionally, no significant differences were observed in treatment persistence over one year when comparing younger or older populations treated with UST, indicating that UST appears safe and effective in the aged PsA population.
References: 1. Smolen et al. EULAR 2020. Abstract #2755; 2. Gossec et al. EULAR 2020. Abstract # 2127.
To cite this abstract in AMA style:
Gossec L, Theander E, Chakravarty S, Bergmans P, Lin I, Noël W, Siebert S, Smolen J. Ustekinumab-Treated Patients with Psoriatic Arthritis in a Real-world Study: Similar Clinical Responses and Treatment Persistence over One Year in Elderly and Younger Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/ustekinumab-treated-patients-with-psoriatic-arthritis-in-a-real-world-study-similar-clinical-responses-and-treatment-persistence-over-one-year-in-elderly-and-younger-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ustekinumab-treated-patients-with-psoriatic-arthritis-in-a-real-world-study-similar-clinical-responses-and-treatment-persistence-over-one-year-in-elderly-and-younger-patients/