Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In contrast to other inflammatory rheumatic diseases, such as rheumatoid arthritis, the therapeutic options in ankylosing spondylitis (AS) with predominant axial manifestations are limited and confined to non-steroidal anti-inflammatory drugs (NSAIDs) and, if this treatment fails, to tumour necrosis factor α blockers. Ustekinumab – a fully human monoclonal antibody against interleukin (IL)-12 and -23 – has been shown to be effective in psoriasis and is currently in phase 3 trials in psoriatic arthritis and Crohn’s disease. The purpose of the current study was to investigate the short-term efficacy and safety of ustekinumab in patients with active AS.
Methods: In this prospective, open-label, proof-of-concept clinical trial (ClinicalTrials.gov identifier NCT01330901), ustekinumab in a dose of 90 mg was administered subcutaneously at baseline, week 4 and week 16 in 20 patients with active AS. Eligible patients were required to have a diagnosis of AS according to the modified New York criteria and an active disease defined as a BASDAI score of ≥ 4 despite previous NSAIDs treatment. The primary study endpoint was the proportion of patients with ASAS40 response at week 24.
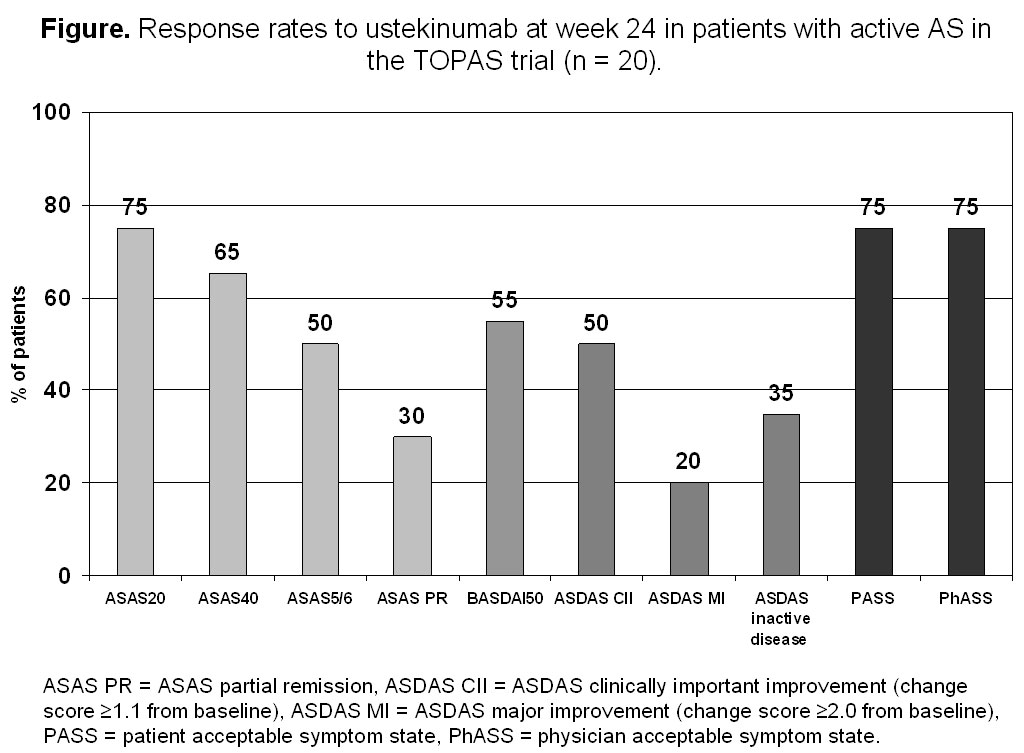
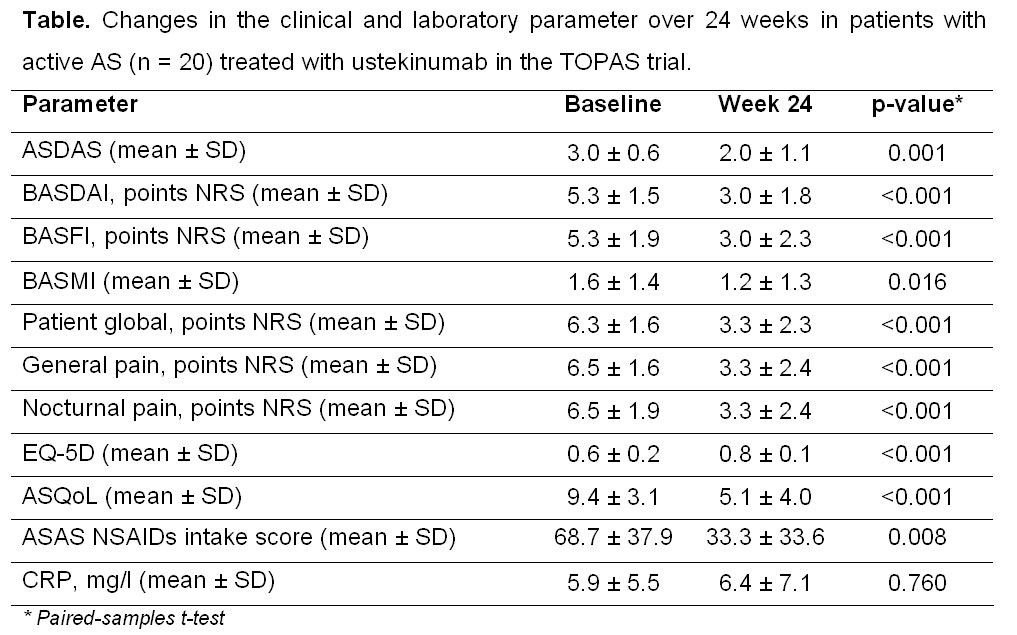
Results: At week 24, ASAS40 response was reached by 65% of the patients. ASAS partial remission and a ≥50% improvement of the BASDAI were achieved in 30% and 55% of the patients, respectively. Most of the other outcome parameters also showed a clear and significant improvement (figure, table). There was no reduction of serum C-reactive protein level in the whole group, however, a clear CRP reduction occurred in the ASAS40 responder group (-1.1±7.5 mg/l) vs. the non-responder group (+3.3±3.5 mg/l), p = 0.008. Overall, ustekinumab was well tolerated: only one serious adverse event (AS worsening) was observed, there were no adverse events leading to study discontinuation, no cases of serious infections, malignancies or deaths.
Conclusion: In this prospective, open-label, proof-of-concept clinical trial, ustekinumab treatment seems to be effective with a significant reduction of signs and symptoms of active AS. For comparison, the ASAS40 response rates in similarly designed AS trials with methotrexate [1], abatacept [2], or anakinra [3] were 10%, 13%, or 20%, respectively.
References: 1. Haibel H, et al. Ann Rheum Dis 2007;66:419-21. 2. Song IH, et al. Ann Rheum Dis 2011;70:1108-10. 3. Haibel H, et al. Ann Rheum Dis 2005; 64:296-8.
Disclosure:
D. Poddubnyy,
MSD,
5,
AbbVie, MSD, Pfizer, and USB,
8;
J. Callhoff,
None;
J. Listing,
None;
J. Sieper,
AbbVie, Merck, Pfizer, UCB,
2,
AbbVie, Merck, Pfizer, UCB,
5,
AbbVie, Merck, Pfizer, UCB,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/ustekinumab-for-the-treatment-of-patients-with-active-ankylosing-spondylitis-results-of-a-28-week-prospective-open-label-proof-of-concept-study-topas/