Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: While clinical trials have demonstrated the efficacy of upadacitinib (UPA), real-world data are essential to understand patient characteristics and assess its effectiveness in routine clinical practice.This study aims to describe a real-world cohort of patients with rheumatoid arthritis (RA) initiating treatment with UPA, analyzing cohort characteristics, UPA efficacy, and its glucocorticoid (GC)-sparing effect.
Methods: This was a multicenter observational study of patients with active RA who started UPA. Prescription decisions were made at the discretion of rheumatologists, and patients were included consecutively. Epidemiological and clinical data were collected. Concomitant GC treatment was assessed at baseline and at follow-up visits after 1, 3, 6, and 12 months.
Results: A total of 99 patients were included, 89.9% of whom were female, with a mean (SD) age of 55.17 (12.0) years. Among them, 19.2% were ≥65 years old. At UPA initiation, patients generally had moderate to high disease activity, with a mean (SD) DAS28 score of 4.75 ± 1.5. UPA was used in combination with csDMARDs in 53.5% of patients and with GCs in 69.7%, with a mean (SD) dose of 7.37 ± 5.0 mg/dL. Seventy percent of patients started UPA as ≥3rd-line advanced therapy (additional data in Table 1). At 3, 6, and 12 months, 82%, 71%, and 61% of patients continued UPA treatment, while 60.5%, 65%, and 67% had achieved low disease activity or remission, respectively.A GC-sparing effect was observed throughout the study period. At baseline, nearly 70% of patients were receiving GC therapy. By 3 months, 20% had discontinued GC, and by 12 months, only 33% remained on GC. The mean GC dose also decreased (Table 2).A secondary analysis compared patients who started UPA as ≥3rd-line advanced therapy (D2T-RA) with those who initiated it as 1st or 2nd-line treatment. Patients in the ≥3rd-line group had significantly longer disease duration (15.1 vs. 9.58 years, p=0.005) and had received more csDMARDs before starting advanced therapy. However, they were more likely to start UPA as monotherapy (45% in D2T-RA vs. 70% in non-D2T-RA). No significant differences were observed in baseline disease activity or GC use. Treatment response and GC-sparing effects were similar in both groups.Among patients ≥65 years old, combination therapy with csDMARDs was used in 50% of patients, and 65% received GC, with a mean (SD) dose of 5.29 ± 3.0 mg/d. Treatment response and GC-sparing effects were comparable to those in patients ≤65 years old. Over the follow-up period, nine infections were reported, including two severe cases. UPA was discontinued in two patients.
Conclusion: Most patients in this real-world cohort initiated UPA as ≥3rd-line advanced therapy. Half started UPA in combination with csDMARDs, and 70% received concurrent GC therapy. A significant early reduction in GC use was observed, with over half of GC-treated patients discontinuing GC by 12 months. UPA was effective in both older patients and those with refractory disease.
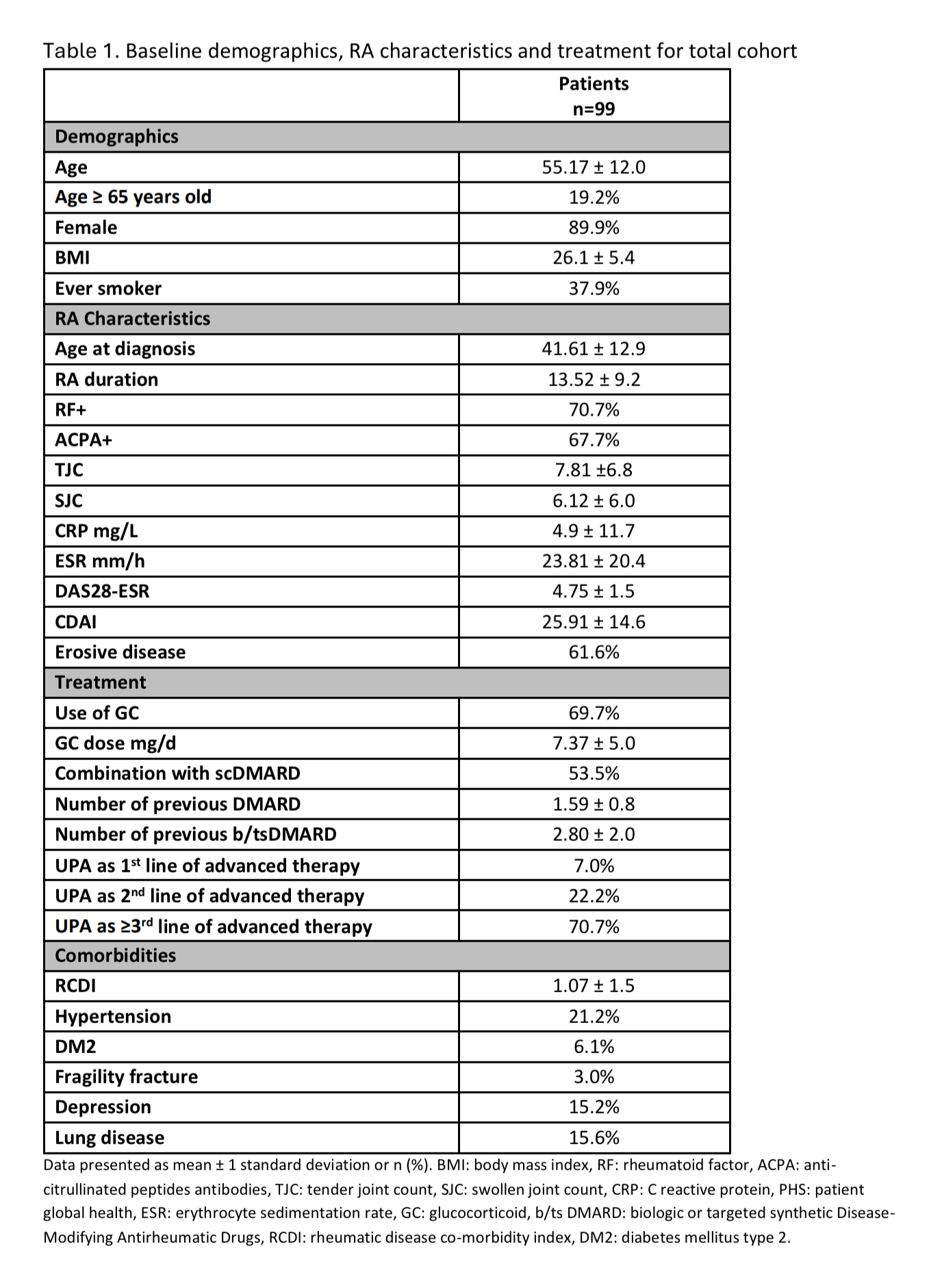 Data presented as mean ± 1 standard deviation or n (%). BMI: body mass index, RF: rheumatoid factor, ACPA: anti-citrullinated peptides antibodies, TJC: tender joint count, SJC: swollen joint count, CRP: C reactive protein, PHS: patient global health, ESR: erythrocyte sedimentation rate, GC: glucocorticoid, b/ts DMARD: biologic or targeted synthetic Disease-Modifying Antirheumatic Drugs, RCDI: rheumatic disease co-morbidity index, DM2: diabetes mellitus type 2.
Data presented as mean ± 1 standard deviation or n (%). BMI: body mass index, RF: rheumatoid factor, ACPA: anti-citrullinated peptides antibodies, TJC: tender joint count, SJC: swollen joint count, CRP: C reactive protein, PHS: patient global health, ESR: erythrocyte sedimentation rate, GC: glucocorticoid, b/ts DMARD: biologic or targeted synthetic Disease-Modifying Antirheumatic Drugs, RCDI: rheumatic disease co-morbidity index, DM2: diabetes mellitus type 2.
.jpg) Evolution since baseline and up to 12 months of follow up of: A DAS28, B proportion of patients in LDA (low disease activity)/ REM (remission), C proportion of patients in treatment with GC and D mean dose of GC, in de entire cohort (full line), D2T-RA (dashed line) and patients ≥65 years old (dotted line).
Evolution since baseline and up to 12 months of follow up of: A DAS28, B proportion of patients in LDA (low disease activity)/ REM (remission), C proportion of patients in treatment with GC and D mean dose of GC, in de entire cohort (full line), D2T-RA (dashed line) and patients ≥65 years old (dotted line).
To cite this abstract in AMA style:
Ruiz-Esquide V, Mateo L, Pérez García C, Sang-Park H, Holgado S, Aparicio M, Frade B, Sarmiento-Monroy J, Nack A, Ramirez Garcia F, Gomez-Puerta J, Sanmartí R. Use of Upadacitinib in Real-World Clinical Practice: Patient Characteristics and Glucocorticoid-Sparing Effect [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-upadacitinib-in-real-world-clinical-practice-patient-characteristics-and-glucocorticoid-sparing-effect/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-upadacitinib-in-real-world-clinical-practice-patient-characteristics-and-glucocorticoid-sparing-effect/

.jpg)