Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Multiple small studies have demonstrated that patients with rheumatic disease can and are willing to use smartphones to track disease severity. The objective of this study is to examine the engagement of an app that allows collection of PRO and passive phone data, in terms of adherence, flares, timing and predictors of discontinuation.
Methods: Participants were enrollees in Forward, The National Databank for Rheumatic Diseases; 700 were invited in two phases. In Phase I,2013-2014, 190 patients enrolled, downloaded the app and agreed to respond to daily PROs (pain and function VAS) for the initial 3months and weekly PROs for the duration (PAS-II). In Phase I, flare questions were triggered if the daily ΔVAS≥2.5 or weekly ΔPAS-II≥1.67. Phase II had 256 participants in 2014-2015 with identical study design but biweekly flare assessment irrespective of PRO.
Correct adherence was computed daily and weekly over the study period to the point of discontinuation defined as no app use ≥ month (implementation). Kaplan Meier estimate was used to analyze time to discontinuation and Cox regression for predictors. Confounders included sociodemographic, clinical, passive phone behavior, and seasonal factors. Kappa statistics between PRO and flares were computed to measure agreement.
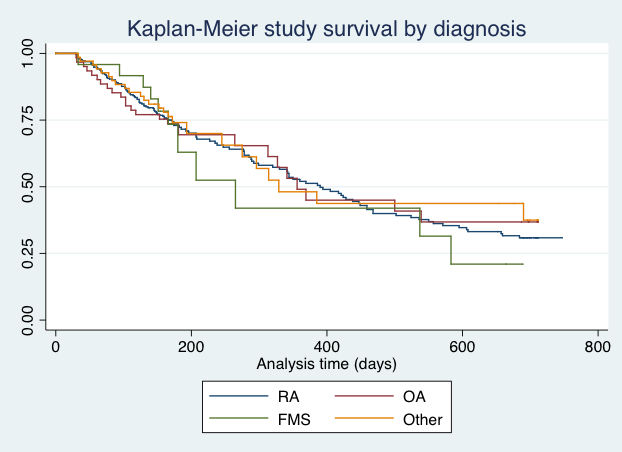
Results: Of 446 patients, 66% had RA, 89% were female, and baseline age 53 yrs (SD 11) and HAQ-II 1.0 (0.7). Overall, 62±28% of patients reported daily measures in the app. Daily and weekly correct implementation was 68±40% and 90±40%. The probability of discontinuation at 6 months was 0.78 and 1 year, 0.64.No differences were found by diagnosis (P log ranks 0.92) (Figure).Younger age, pain, and HAQ were significant predictors of discontinuation. Half of Phase I patients reported having ≥ 1 flare (IQ 0-3), and Phase II reported≥3(0-6) flares. By phase, the agreement between flares and PROs was 55% and 65%.
Conclusion: This study revealed good levels of correct adherence which holds promise for passive behavior use in clinical follow-up and patient self-management. Examining predictors of discontinuation of response to smartphone data collection is also important as additional reminders can encourage continued participation.
Table. HR (95%CI) using both definitions in the overall sample.
|
|
Adherence (90 days) |
Implementation |
Implementation |
Persistence at x days |
|
|
Stats |
Daily |
Daily |
Weekly |
x days |
|
|
Mean (SD) |
61.5 (28.0) |
68.0 (24.0) |
89.8 (40.1) |
180 |
77.9% |
|
Median (Q1-Q3) |
62.0 (43.0 – 89.0) |
69.0 (53.0 – 90.0) |
97.0 (80.0 – 100.0) |
360 |
63.9% |
|
Phase 1 |
|
|
|
|
|
|
Mean (SD) |
44.2 (19.4) |
69.3 (25.0) |
71.6 (34.0) |
180 |
72.1% |
|
Median (Q1-Q3) |
47.8 (32.2 – 60.0) |
74.2 (55.8) |
84.5 (53.6 – 97.2) |
360 |
50.5% |
|
Phase 2 |
|
|
|
|
|
|
Mean (SD) |
71.3 (27.8) |
73.5 (25.1) |
78.5 (50.6) |
180 |
73.7% |
|
Median (Q1-Q3) |
80.0 (55.5 -93.3) |
81.1 (57.8 – 93.4) |
95.1 (33.5 – 100.0) |
360 |
. |
To cite this abstract in AMA style:
Michaud K, Pedro S, Grinnell M, Schumacher R. Use of Smartphones in Collecting Patient Reported Outcomes (PROs): Feasibility of a Study Nested in a US National Cohort [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/use-of-smartphones-in-collecting-patient-reported-outcomes-pros-feasibility-of-a-study-nested-in-a-us-national-cohort/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-smartphones-in-collecting-patient-reported-outcomes-pros-feasibility-of-a-study-nested-in-a-us-national-cohort/