Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: NSAIDs are commonly used for OA pain, but the benefits of pain relief must be carefully weighed against the potential risk for gastrointestinal (GI), cardiovascular (CV), and renal adverse events (AEs). Increased risk for these AEs changes the risk-benefit profile of certain NSAIDs such that they are not recommended by clinical treatment guidelines. However, information for real-world NSAID use despite these recommendations is lacking. This study aimed to evaluate the use of prescription NSAIDs among patients with hip/knee OA for whom specific NSAID treatment is not recommended per clinical treatment guidelines.
Methods: This retrospective cohort study used the IBM MarketScan® claim database (July 2016–June 2019). Adult patients with a diagnosis of hip/knee OA between July 2017 and June 2018 (index date) were identified. If more than one diagnosis was available, one was randomly selected as the index date. Patients were required to have 1 year of continuous enrollment before the index date (baseline period) to establish their baseline risks (high, moderate, or low) for NSAID-related GI, CV, and renal AEs, respectively (table 1), and were categorized into 6 risk profiles based on clinical guidelines (table 2). Patients were followed post-index to assess specific prescription NSAID use that was not recommended per the patient’s risk profile, and Kaplan–Meier survival analyses were used to estimate 1-year cumulative incidence rates.
Results: Among 218,349 adults with hip/knee OA (mean age ± standard deviation, 60.8 ± 11.1 years; 61.9% female; 74.4%/25.6% commercial/Medicare), 65.9% (n=143,833) had no evidence of increased risk. Therefore, for these patients, there was no guideline recommendation against NSAID use (table 2). Among the remaining patients (n=74,516; 34.1%), the 1-year rate of NSAID use that was not consistent with guideline recommendations was 49.1% overall and ranged from 33.2%–63.8% for the different risk profiles (table 3). The subgroup of patients at high risk of NSAID-related GI AEs and at low risk of NSAID-related CV AEs (6.9% of the study population) had the highest 1-year rate of NSAID use that was not consistent with guideline recommendation (63.8%; table 3).
Conclusion: This study showed that in the real-world setting, over one-third of patients with hip/knee OA were found to have risk factors that could increase their risk for NSAID-related AEs. Approximately half of these patients had evidence of prescription NSAID use that was inconsistent with guideline recommendations, suggesting the lack of reliable pain medications for OA patients. These results emphasize the need to consider the risks associated with NSAIDs and to individualize OA management strategies to mitigate the risk of NSAID-related AEs. This study was limited by the lack of information on the use of over-the-counter NSAIDs and drugs that would decrease the risk of NSAID-related AEs, as well as by the inability to distinguish between short-term and long-term prescription NSAID use.
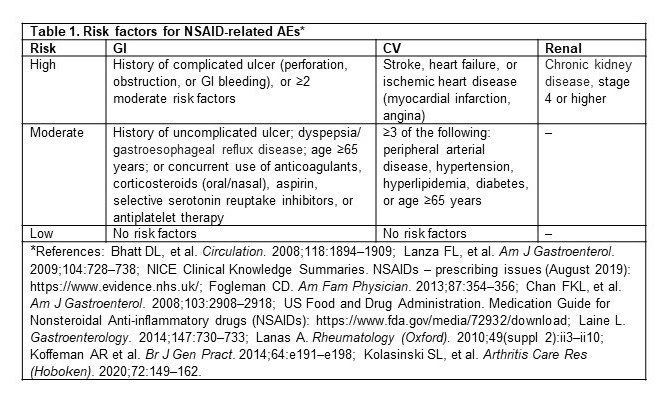 Table 1. Risk factors for NSAID-related AEs*
Table 1. Risk factors for NSAID-related AEs*
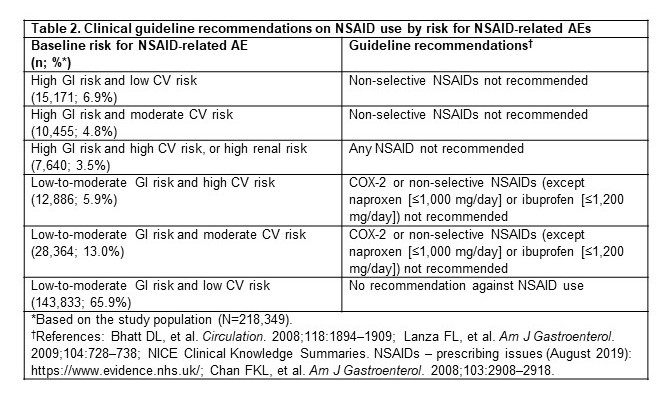 Table 2. Clinical guideline recommendations on NSAID use by risk for NSAID-related AEs
Table 2. Clinical guideline recommendations on NSAID use by risk for NSAID-related AEs
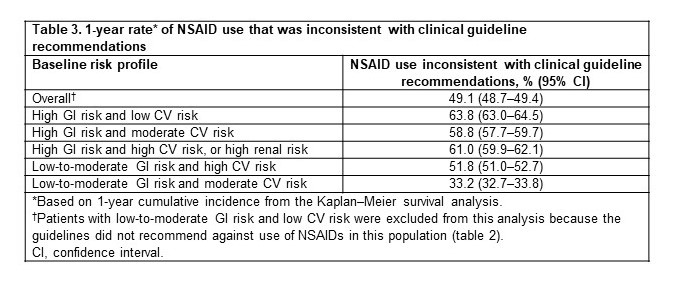 Table 3. 1-year rate* of NSAID use that was inconsistent with clinical guideline recommendations
Table 3. 1-year rate* of NSAID use that was inconsistent with clinical guideline recommendations
To cite this abstract in AMA style:
Patel J, Wei W, Bannuru R, Iyer R, Shaikh N, LeMasters T, Iloabuchi C, Wang D, Sambamoorthi U. Use of Prescription Nonsteroidal Anti-inflammatory Drugs (NSAIDs) in Adults with Hip/Knee Osteoarthritis (OA) at Increased Risk for NSAID-related Adverse Events [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/use-of-prescription-nonsteroidal-anti-inflammatory-drugs-nsaids-in-adults-with-hip-knee-osteoarthritis-oa-at-increased-risk-for-nsaid-related-adverse-events/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-prescription-nonsteroidal-anti-inflammatory-drugs-nsaids-in-adults-with-hip-knee-osteoarthritis-oa-at-increased-risk-for-nsaid-related-adverse-events/
