Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Although most healthcare professionals are convinced of the quality, safety, and favorable economic impact of biosimilars, their routine use is not yet widespread. MSB11456 is the first tocilizumab biosimilar approved for both IV and subcutaneous administration. Its biosimilarity to the originator has been demonstrated in an extensive non-clinical and clinical program in patients with moderate-to-severe rheumatoid arthritis (RA).The aim of RUBY, a multinational, prospective non-interventional study, is to evaluate the use of MSB11456 (either in initiation or switch) in RA patients over 12 months, assess persistence rate at 6 months after treatment initiation, and identify factors associated with treatment persistence for at least 12 months.
Methods: Adult RA patients were included; those participating in any interventional clinical trial were excluded. The primary endpoint was the persistence on MSB11456 by 6 months after treatment initiation. Data collected included basic clinical characteristics, inflammatory disease history, current clinical status including disease activity score (DAS28-CRP), and adverse events at each follow-up visit up to 12 months. Data are presented using descriptive statistics. A logistic regression model will be employed to identify potential predictive factors of persistence at 12 months. We present here patient baseline characteristics, and 6-months intermediate results.
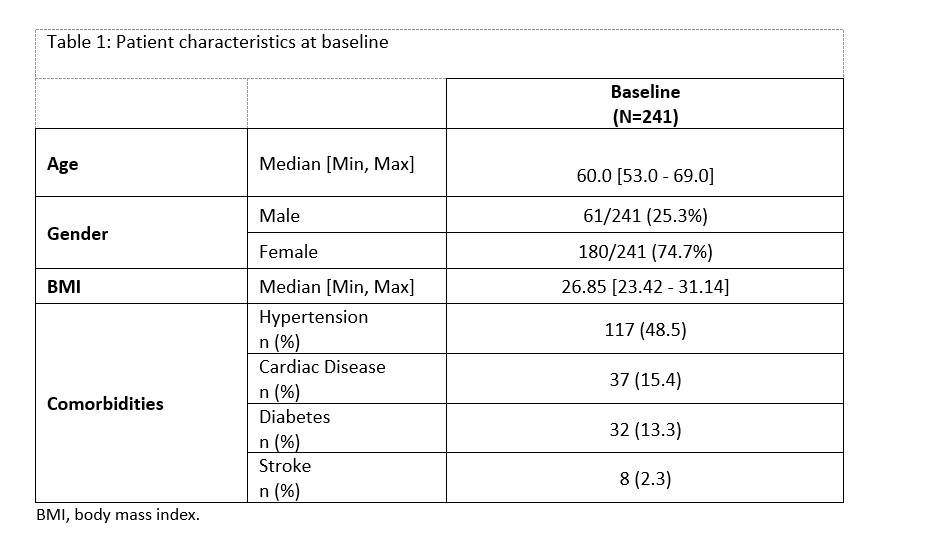
Results: 241 RA patients in Germany (219), UK (17) and Spain (5) completed the 6-month follow-up visit. At MSB11456 initiation, the median duration of RA was 10.5 years. 59.3% patients were naïve to tocilizumab, whereas 40.7 % switched from the originator. Patient characteristics at baseline, are summarized in Table 1. The most frequent comorbidity was hypertension (48.5%) and most frequent extra-articular manifestations were rheumatoid nodules (9.1%), Raynaud’s phenomenon (2.1%) and pulmonary fibrosis (2.1%). MSB11456 was administered as monotherapy in 52.3% patients, mainly by subcutaneous route (78%) using often the autoinjector. DAS28-CRP disease activity improved at 6 months, with high rates of low-disease activity and remission (Table 2). Corticosteroids (CS) dose decreased in 28/82 (34.2%) tocilizumab-naïve patients (in which 13 patients < 5 mg/day or discontinuing CS). By 6 months, 127 patients (53%) experienced at least one adverse event (AE), of which 66 (27%) had AE(s) possibly attributable to MSB11456 that led to permanent discontinuation in 22 (9%) patients At 6 months, 86% of patients remained on MSB11456. Among patients with comorbidities, no worsening in existing conditions was observed, with high persistence rates (86.5% to 100%) (Table 3).
Conclusion: The RUBY study evaluates the real-world use of the first tocilizumab biosimilar to treat RA. Results at 6-months demonstrate high persistence on treatment with improvement in RA disease activity and no worsening of cardiovascular comorbidities. Further study will examine factors influencing treatment persistence at 12 months in clinical practice.
To cite this abstract in AMA style:
Kay J, feist E, Choy E, Kaufmann J, Wong E, Corominas H, Flipo R, Avouac J, López Lasanta M, Monnet J, Baker P, Romanova Michailidi M, Dolfi F, Pourcel G. Use of (MSB11456), the first tocilizumab biosimilar approved for rheumatoid arthritis: 6-month data from an international observational study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-msb11456-the-first-tocilizumab-biosimilar-approved-for-rheumatoid-arthritis-6-month-data-from-an-international-observational-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-msb11456-the-first-tocilizumab-biosimilar-approved-for-rheumatoid-arthritis-6-month-data-from-an-international-observational-study/

.jpg)
.jpg)