Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Medium- and small-vessel vasculitides (MVV and SVV) are a heterogeneous group of inflammatory diseases affecting the vascular wall. Current treatment strategies are based on a combination of glucocorticoids (GCs) and disease modifying anti-rheumatic drugs (DMARDs), but relapses, GC dependence and treatment-related toxicity remain major challenges. In preclinical studies, the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway has emerged as a key regulator of chronic vascular inflammation. To date, data on the use of JAK inhibitors (JAKi) in clinical practice remains limited. This study aimed to evaluate the off-label use of JAKi in MVV and SVV, focusing on patient characteristics, therapeutic efficacy, and safety outcomes in multiple centers worldwide.
Methods: This was a retrospective, multicenter, observational study that collected real-world data on adult patients who met the classification criteria for MVV or SVV and were treated with JAKi. Data were extracted from electronic medical records. Complete response (CR) was defined as BVAS =0 and prednisone ≤5 mg/day, partial response (PR) as BVAS =0 and prednisone between 6 and 10 mg/day, and active vasculitis as BVAS≥1 regardless of prednisone dose.
Results: Fourteen patients (6 granulomatosis with polyangiitis, 2 microscopic polyangiitis, 3 polyarteritis nodosa, 2 cryoglobulinemic vasculitis, and 1 IgA vasculitis) from 8 different centers were included (Table). The median age was 57 (IQR 49-68) years. JAKi were prescribed for refractory disease in 12/14 (85.7%) and for GC sparing in 4/14 (28.6%). The median disease duration prior to JAKi initiation was 2.4 years (IQR 0.6-3.1). Among the JAKi, tofacitinib and upadactinib were used in 6/14 (42.85%) and baricitinib in 2/14 (14.3%) patients, respectively. At JAKi initiation, 13/14 (92.8%) patients were still receiving GCs at a median prednisone dose of 13.75 mg/day (IQR 7.5-20). JAKi were prescribed alone in 8/14 (47.1%) patients, in combination with conventional synthetic DMARDs in 4/14 (28.6%) and with biologic DMARDs in 2/14 (14.3%). After a median follow-up of 7.4 months (IQR 4.1-26.6), JAKi achieved an overall response in 11/14 (78.6%) patients (7 CR and 4 PR). Three patients (21.4%) relapsed while on JAKi, including a major relapse that led to discontinuation of JAKi in one patient. Two other patients discontinued JAKi due to adverse events (1 hepatic toxicity and 1 venous thromboembolism). Twelve infections occurred in 5/14 (35.7%) patients, including 6 viral (among which 4 SARS-CoV-2 infections) and 6 bacterial infections. Of the 12 infections, 8 occurred with upadactinib, 3 with tofacitinib and 1 with baricitinib. At the last follow-up, 11/14 (78.6%) were still taking JAKi.
Conclusion: JAK inhibitors may offer a promising therapeutic alternative for refractory vasculitis. However, the risk of adverse events, especially infections, emphasizes the need for careful patient selection and prophylactic measures. Further prospective studies are needed to define the efficacy, safety and optimal role of JAKi in the treatment of MVV and SVV.
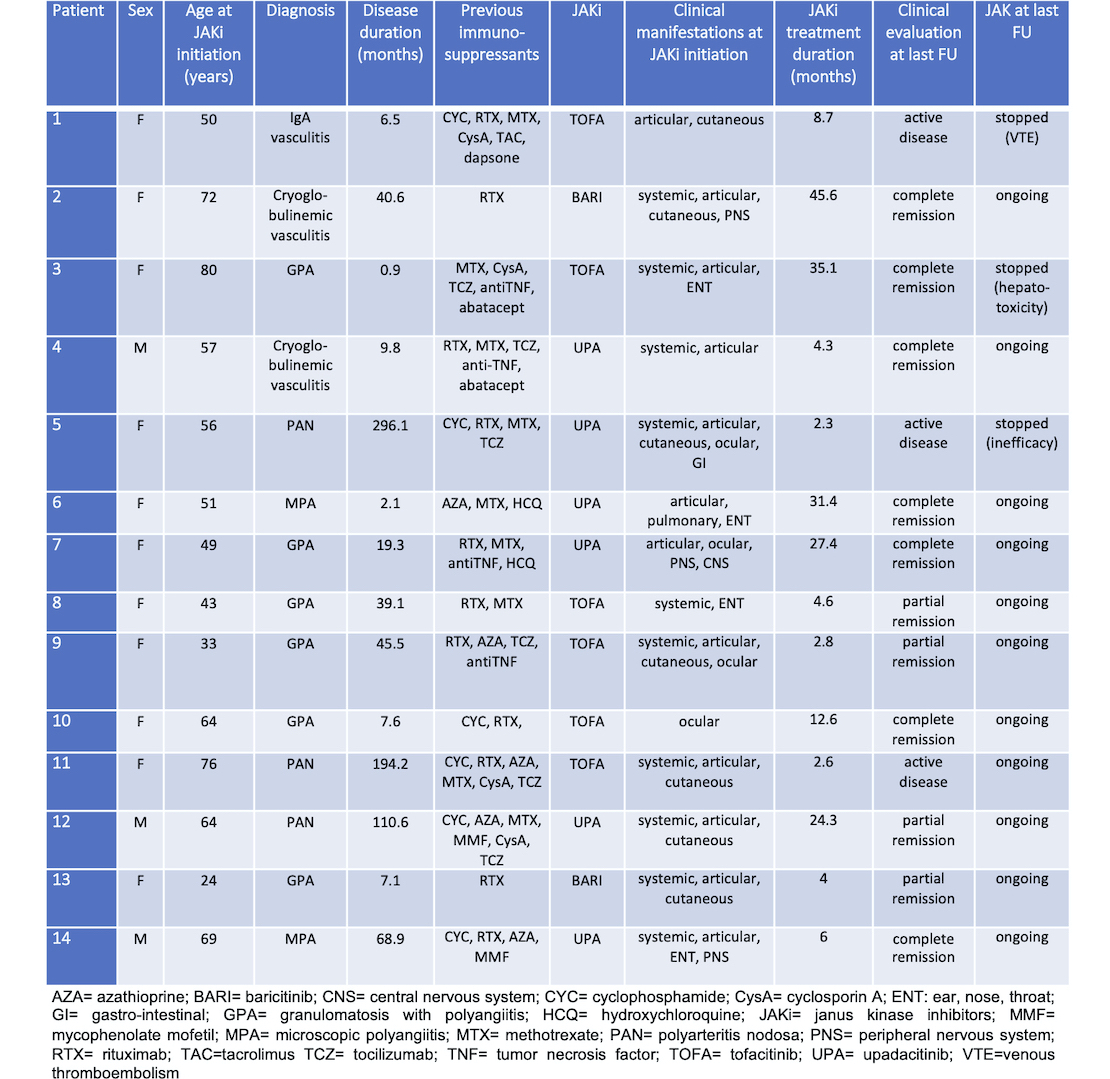 Table. Demographic and clinical features of the 14 patients with small- to medium-sized vessel vasculitis treated with JAKi
Table. Demographic and clinical features of the 14 patients with small- to medium-sized vessel vasculitis treated with JAKi
To cite this abstract in AMA style:
ricordi c, treppo e, Novikov P, Russo P, moroni L, Fassio A, Delvino P, lobbes h, Marvisi C, Muratore F, Puéchal X, Salvarani C, Terrier B. Use of JAK Inhibitors in Medium- and Small-Sized Vasculitides: a Retrospective Multicenter Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/use-of-jak-inhibitors-in-medium-and-small-sized-vasculitides-a-retrospective-multicenter-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-jak-inhibitors-in-medium-and-small-sized-vasculitides-a-retrospective-multicenter-study/
