Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Biologic (b) and targeted synthetic (ts) DMARDs used in the management of rheumatoid arthritis (RA) target inflammatory pathways implicated in the pathogenesis of multiple myeloma (MM). Yet little is known about the association between use of bDMARDs or tsDMARDs in RA and the incidence of MM. Our objective was to estimate the associations between b/tsDMARD use and the risk of MM among persons with RA using Veterans Health Administration (VHA) data.
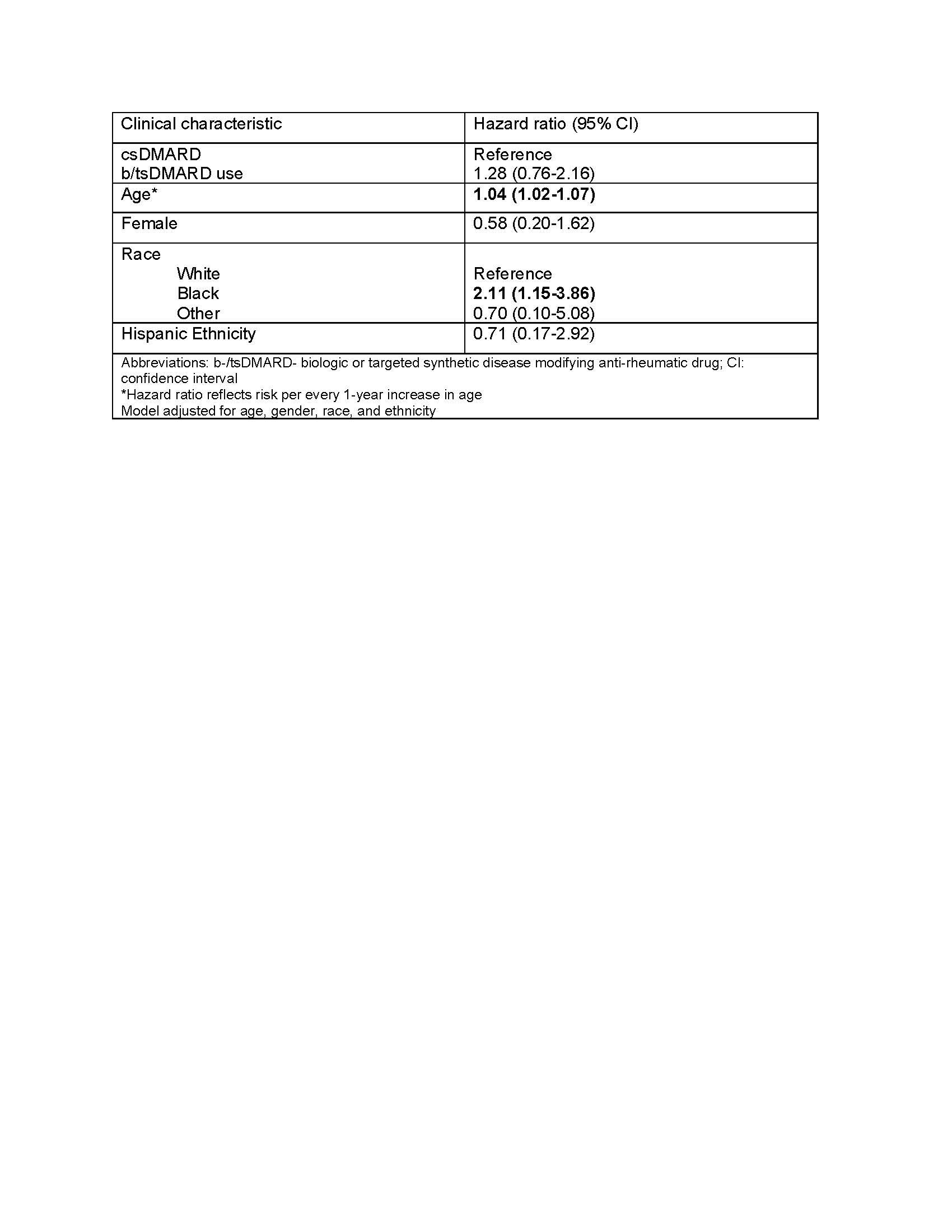
Methods: In this retrospective cohort study, we identified patients >18 years of age diagnosed with RA in any United States VHA facility from 1/1/2002 and 12/31/2018. Diagnosis of RA was defined with use of two or more International Classification of Diseases Version 9 or 10 (ICD9 or ICD10) codes for RA at least 7 days apart but no more than 365 days apart and a prescription for a csDMARD within 90 days of the first RA diagnosis (index date). Medication data was derived from the outpatient prescription fills, bar coded medication administration (BCMA), and intravenous (IV) data domains. The csDMARDs included in these analyses were: methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine. The bDMARDs included were tumor necrosis factor inhibitors (TNFi) and non-TNFi biologics such as tocilizumab, rituximab, abatacept, and biosimilars; tsDMARD was tofacitinib. Patients with MM before the diagnosis of RA were excluded. Incident MM was determined by 1 or more ICD9/10 code or ICD-oncology codes. Multivariable Cox proportional hazards model with time-varying exposure was performed to estimate the hazard ratio for developing MM among those during and following the use of a b/tsDMARD relative to b/tsDMARD-naïve persons adjusting for age, gender, race, and ethnicity.
Results: 27,540 veterans with RA met study eligibility criteria, of whom 8,322 (30%) had ever taken a b/tsDMARD during follow up. Over the study period there were 77 incident MM over a total of 192,000 person years (median follow-up 5.8 years). There were 55 events in users of csDMARDs, an incidence rate (IR) of 0.40 (95% CI 0.30-0.52) per 1000 person-years and 22 in persons currently or formerly using b/tsDMARDs (IR 0.41, 0.25-0.61 per 1000 person years). The unadjusted hazard ratio for MM following b/tsDMARD use relative to csDMARD only use was 1.04 (0.63, 1.73), which increased to 1.28 (0.76, 2.16) after adjusting for demographic characteristics (Table 1).
Conclusion: In this nationwide VA study, rates of MM were not different among b/tsDMARD and csDMARD users after accounting for demographics. Of note, the median interval from initiation of a bDMARD to the end of follow-up was approximately 5.8 years, which does not allow for an examination of a possible longer-term influence.
To cite this abstract in AMA style:
Singh N, Peterson A, Baraff A, Chung S, Coffey D, England B, Reid P, Baker J, Barton J, Smith N, Mikuls T, Weiss N. Use of Disease Modifying Anti-rheumatic Drugs and Risk of Multiple Myeloma in US Veterans with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/use-of-disease-modifying-anti-rheumatic-drugs-and-risk-of-multiple-myeloma-in-us-veterans-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-disease-modifying-anti-rheumatic-drugs-and-risk-of-multiple-myeloma-in-us-veterans-with-rheumatoid-arthritis/