Session Information
Session Type: Abstract Session
Session Time: 2:00PM-2:15PM
Background/Purpose: Tumor necrosis factor inhibiting (TNFi) therapies are associated with new-onset psoriasis in children with inflammatory bowel disease (IBD) and juvenile idiopathic arthritis (JIA). We aimed to compare the differential effect of three TNFi therapies and to examine whether the addition of a nonbiologic disease-modifying anti-rheumatic drug (DMARD) impacts the incidence of psoriasis in children with IBD and JIA.
Methods: This was a single-center retrospective cohort study of electronic health record data from 2008 to 2020. Inclusion criteria were: 1) 2 ICD-9 or ICD-10 codes for JIA, IBD, or chronic nonbacterial osteomyelitis (CNO); 2) age at diagnosis of under 19 years; 3) at least 2 visits with a study center rheumatologist or gastroenterologist. Subjects with psoriasis prior to the diagnosis of JIA, IBD, or CNO were excluded. TNFi exposure was defined as at least 1 prescription for infliximab, etanercept, or adalimumab (ever/never). DMARD exposure was dichotomized as ever/never and included methotrexate. The primary outcome was incident psoriasis defined as the first ICD-9/10 code for psoriasis during a visit with a study center rheumatologist, gastroenterologist, or dermatologist. Incidence rates (IRs) were calculated and stratified by underlying diagnosis, TNFi agent, and DMARD use. IRs for CNO were not calculated due to small sample size.
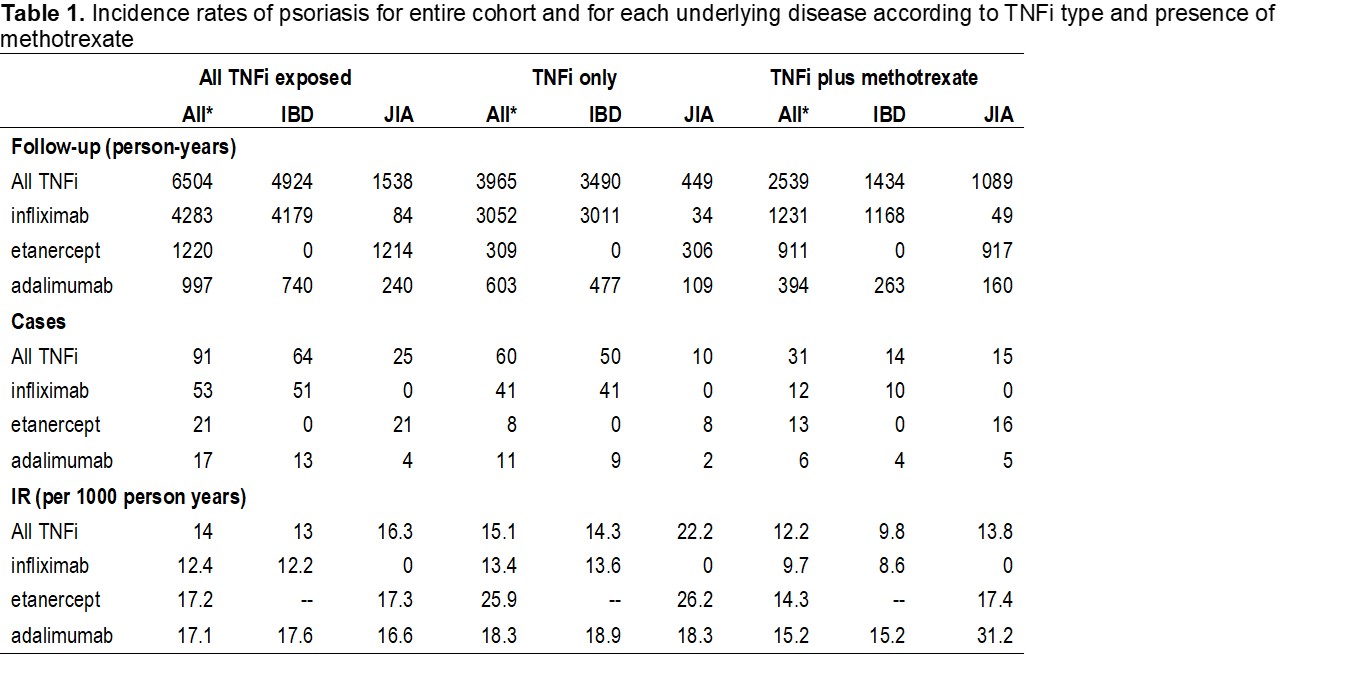
Results: 5088 children met inclusion criteria – 3799 (75%) had IBD, 1185 (23%), had JIA, and 104 (2%) had CNO. 1335 (66%), 304 (15%), and 382 (19%) of children were prescribed infliximab, etanercept, or adalimumab, respectively. 1430 (71%) and 593 (29%) had TNFi exposure with or without a DMARD, respectively. The number of follow-up years, cases, and IRs for the entire cohort and for each diagnosis are presented in Table 1. The IR for psoriasis was higher in patients with JIA than IBD in both the TNFi alone and TNFi plus DMARD groups with a risk difference of 7.9 and 4, respectively. Amongst all patients, the IR for psoriasis was lowest for infliximab (12.4) and similar in etanercept and adalimumab (17.1 and 17.2). The IR for infliximab remained lower than the other 2 TNFi when stratified by DMARD use but differed by underlying diagnosis. There were no IBD patients treated with etanercept and of the limited number of JIA patients treated with infliximab (N=21), none developed psoriasis. The IR of psoriasis was lower amongst children on a TNFi who were DMARD-exposed (12.2) versus those unexposed (15.1).
Conclusion: The IR of TNFi-induced psoriasis was lowest for patients exposed to infliximab, even after stratifying by DMARD exposure. For patients with exposure to any of TNFi evaluated, the IR was lower in those also exposed to DMARD.
IR: incidence rate; IBD: inflammatory bowel disease; JIA: juvenile idiopathic arthritis; CNO: chronic nonbacterial osteomyelitis; TNFi: tumor necrosis factor inhibitor
To cite this abstract in AMA style:
Baggett K, Brandon T, Xiao R, Valenzuela Z, Buckley L, Weiss P. Use of Disease-Modifying Anti-Rheumatic Drug Associated with Lower Incidence of Anti-Tumor Necrosis Factor Induced Psoriasis in Children with Inflammatory Bowel Disease and Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/use-of-disease-modifying-anti-rheumatic-drug-associated-with-lower-incidence-of-anti-tumor-necrosis-factor-induced-psoriasis-in-children-with-inflammatory-bowel-disease-and-juvenile-idiopathic-arthrit/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-disease-modifying-anti-rheumatic-drug-associated-with-lower-incidence-of-anti-tumor-necrosis-factor-induced-psoriasis-in-children-with-inflammatory-bowel-disease-and-juvenile-idiopathic-arthrit/