Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: , Primary Sjögren’s Syndrome (pSjS) is a systemic autoimmune inflammatory disease that primarily affects the lacrimal and salivary glands and is associated with B cell hyper-reactivity and elevated serum concentrations of Blys. Belimumab is approved for the treatment of Systemic Lupus Erythematosus, where B cell mediated inflammation is commonly seen. In this retrospective study, we examined the off-label use of belimumab in a small cohort of pSjS with systemic manifestations. We analyzed clinical endpoints based on questionnaires and patient reported outcomes (PROs) as well as multiple serum biomarkers, individually measured as part of a multi-biomarker disease activity assay (MBDA), used predominantly in RA. Vectra DA is a cumulative score based on the serum concentrations of 12 serum proteins (biomarkers); although the score has been validated for use in RA, several components of the test have been implicated in the pathogenesis of SjS.
Methods: , A retrospective chart review study was conducted at a teaching hospital and nine patients diagnosed with pSjS were identified, as defined by the American-European group consensus criteria. The patient’s age ranged from 26 to 73 (mean: 51). All patients studied (1 male, 8 females) had confirmatory minor salivary gland biopsy (focal score>1), 3/9 (33%) were African American and 6/9 (67%) Hispanic. Of the 9 patients studied, 4 were also treated with monthly belimumab infusions (10 mg/kg), as an add-on treatment to address predominantly extra-glandular manifestation, such as arthritis and fatigue, for approximately 60 weeks. Vectra was done for all patients and clinical improvement was assessed by RAPID-3 and FACIT-F scores during the course of the treatment. Individual biomarker concentrations were compared in the 2 groups using the Wilcoxon Exact test.
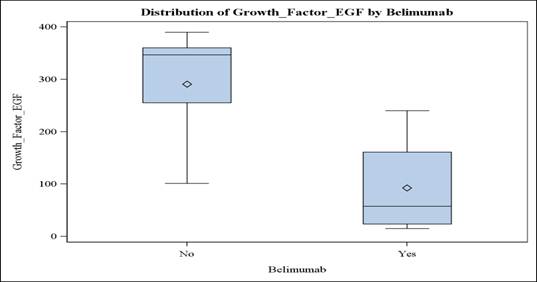
Results: The results for EGF biomarker suggest that there is a statistically significant difference between the underlying distributions of the EGF scores of control group and the EGF scores of Belimumab group (p = 0.03, r=-0.71). The patients on Belimumab had numerically lower serum concentrations of VCAM-1, VEGF-a, IL-6, MMP-3, YKL-40, Leptin, Resistin, SSA, and CRP biomarkers and higher concentrations of the TNF-RI and MMP-1 biomarkers compared to the control group, although the difference did not reach statistical significance. Vectra-DA scores for the belimumab group (mean: 38) and the control group (mean: 31.5) were similar in both groups and were not affected by treatment. Clinical endpoints, such as the RAPID-3 and FACIT scores, did not show a difference between the two groups.
Conclusion: In our pSjS cohort, treatment with Belimumab was associated with significantly lower EGF concentrations, albeit no apparent extra-glandular clinical improvement. Controlled studies and development of more specific disease activity measures are needed.
Disclosure:
S. Bobic,
None;
S. Kadavath,
None;
L. Gerber,
None;
E. Zapantis,
None;
P. V. Efthimiou,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-b-lymphocyte-stimulator-inhibitor-belimumab-may-be-associated-with-a-decrease-in-the-serum-concentration-of-endothelial-growth-factor-in-patients-with-primary-sjogrens-syndrome/