Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Immune checkpoint inhibitors (ICI) antagonize CTLA-4, PD-1, PD-L1, and Lag-3 to stimulate the immune system to treat cancer but may also cause immune-related adverse events (irAEs). These irAEs range from mild to severe, and some such as myocarditis may result in death. The mechanism of action of abatacept (CTLA-4 agonist) may act as the “antidote” to ICI for patients with life-threatening irAEs. Some case reports have reported efficacy of abatacept to treat myocarditis as an irAE and a phase 3 trial is ongoing. We studied the patients who received abatacept for treatment of severe irAEs to describe the cancer characteristics, treatment course, and outcomes of patients receiving this therapy.
Methods: We performed a retrospective case series of patients who received abatacept for treatment of a severe irAE as an adverse effect of ICI at a large tertiary cancer center and healthcare system. We used electronic query to identify all cancer patients who initiated an ICI and who received abatacept (2011-2024). We then confirmed by manual medical record review that abatacept was used for treatment of an irAE. The index date was the initiation of ICI. We collected data on demographics, cancer, cancer treatment, irAEs, irAE treatment, lab abnormalities, and clinical outcomes. We used descriptive statistics to report the data.
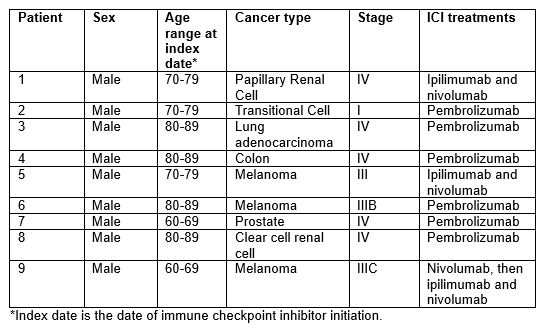
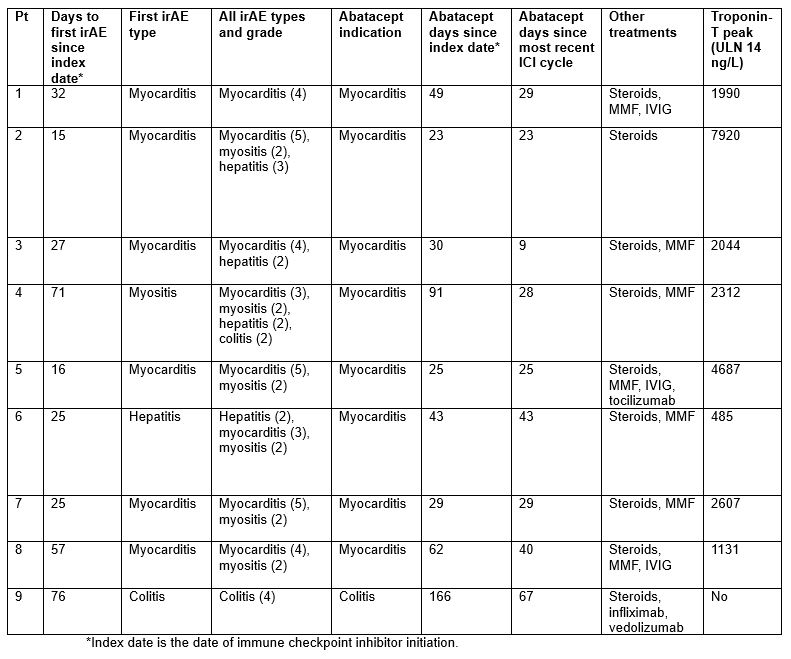
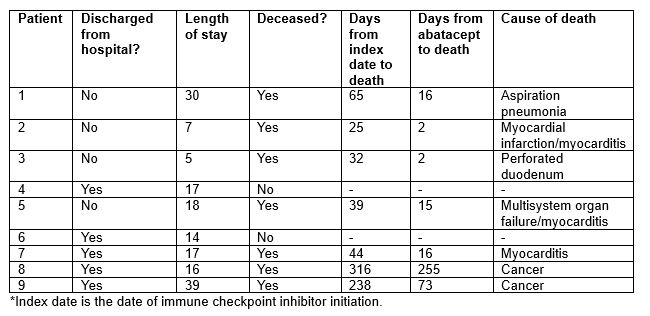
Results: Among 13,028 cancer patients treated with an ICI, we identified 9 patients (0.07%; 7 per 10,000 treated) who received abatacept for the treatment of severe irAE. The mean age was 76.8 years, and all patients were white, non-Hispanic males. The most common cancer type was melanoma (3/9); 6/9 patients received pembrolizumab (Table 1). The median time from index date to first irAE was 27 days. For 7/9 patients, the first irAE was the indication for abatacept. For 8/9 patients, the indication for abatacept was myocarditis; the other patient received abatacept for colitis (Table 2). The mean time from most recent ICI infusion to abatacept was 32.5 days. In addition to abatacept, all patients received steroids, 7 received MMF, and 4 received IVIG. Four patients (44%) died during the hospitalization. Of the 5 who were discharged, 3 have since died. Two out of 9 (22%) patients are currently alive. Of the 7 patients in total who have died, the median time from abatacept to death was 16 days (Table 3).
Conclusion: Use of abatacept for treatment of severe irAE from immunotherapy was very uncommon (7 per 10,000 who initiated ICI). All patients who received abatacept were male, and nearly all received this for myocarditis, with onset soon after ICI initiation. Nearly half died while hospitalized, and only 2/9 are still alive. Additional studies are needed to increase the sample size to draw stronger conclusions. These data highlight the high mortality of myocarditis as an irAE and inform the ongoing trial investigating abatacept.
To cite this abstract in AMA style:
McCarter K, Wang X, Arabelovic S, LeBoeuf N, Buchbinder E, Gedmintas L, MacFarlane L, Rao D, Shadick N, Sharon E, Weber B, Nohria A, Gravallese E, Sparks J. Use of Abatacept for the Treatment of Severe Immune-related Adverse Events from Immune Checkpoint Inhibitors for Cancer: A Case Series [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/use-of-abatacept-for-the-treatment-of-severe-immune-related-adverse-events-from-immune-checkpoint-inhibitors-for-cancer-a-case-series/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-of-abatacept-for-the-treatment-of-severe-immune-related-adverse-events-from-immune-checkpoint-inhibitors-for-cancer-a-case-series/