Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have nephroprotective and cardioprotective benefits and may have a role in lupus nephritis (LN) treatment. 2023 EULAR SLE treatment guidelines indicate that SGLT2i may be considered in patients with LN with reduced glomerular filtration rate (GFR) < 60-90 ml/min/m2 or proteinuria >0.5-1.0 g/day (Fanouriakis et al. Ann Rheum Dis 2024). We sought to assess recent use of SGLT2i and adverse events among patients with LN.
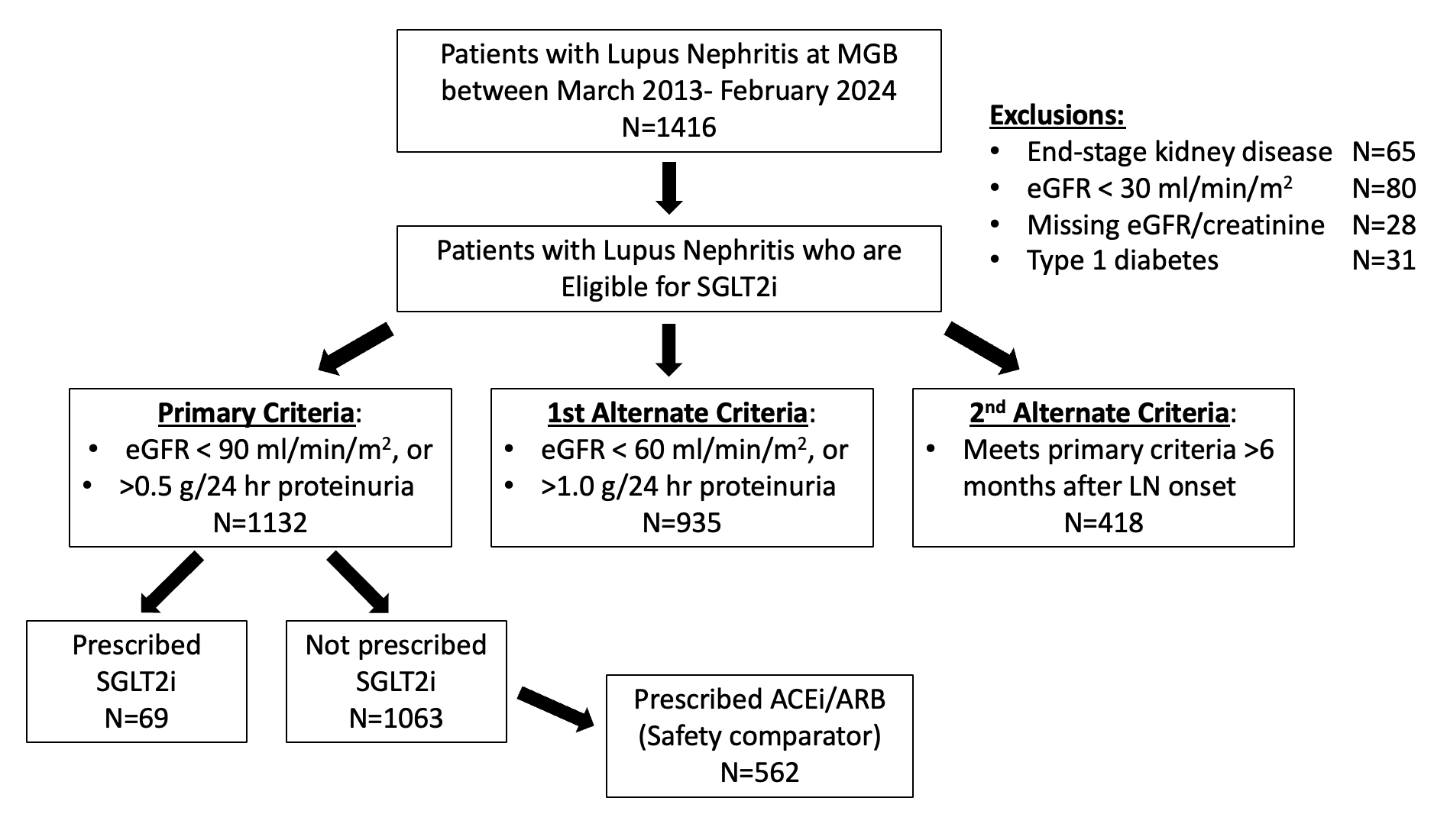
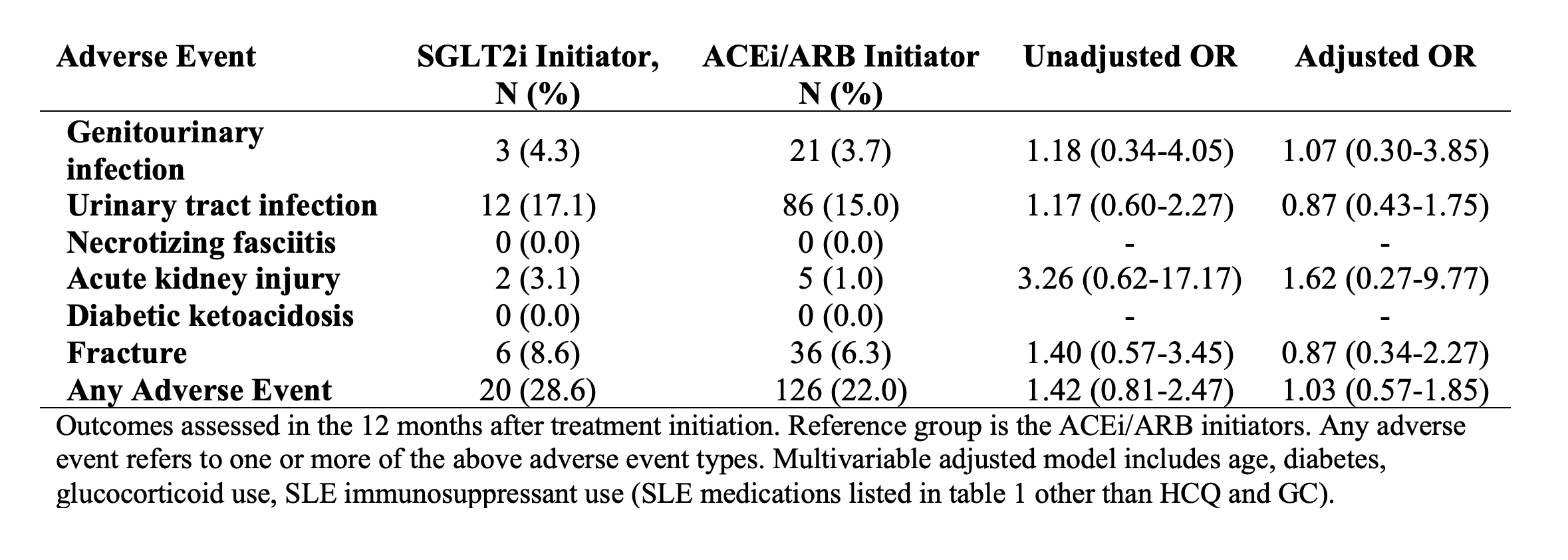
Methods: We utilized data from the US Mass General Brigham (MGB) electronic health record database. We identified all patients with LN (defined as ≥2 codes for SLE >30 days and < 2 years apart and ≥1 LN code ICD-10 M32.14 or M32.15 or ≥2 ICD-9 or 10 nephritis codes) who were eligible to receive an SGLT2i between March 2013 and February 2024: without type 1 diabetes or end-stage kidney disease, with baseline GFR > 30 ml/min/m2, and with GFR below 90 ml/min/m2 and/or proteinuria >0.5 g/day. We also assessed two alternative definitions of indications for SGLT2i: requiring GFR < 60 ml/min/m2 and/or proteinuria >1.0 g/day or requiring GFR < 90 ml/min/m2 and/or proteinuria >0.5 g/day at least six months after LN onset, coinciding with the typical maintenance phase of treatment. We identified all eligible patients with LN who initiated SGLT2i. Among SGLT2i non-users, we identified patients who initiated angiotensin converting enzyme inhibitor or angiotensin receptor blockers (ACEi/ARB). We assessed characteristics at the date of meeting eligibility criteria. We compared the risk of adverse events within 12 months following SGLT2i or ACEi/ARB initiation, including genitourinary infections, acute kidney injury, diabetic ketoacidosis (DKA), and fractures. We used logistic regression, adjusting for age, diabetes, glucocorticoids, and SLE immunosuppressant use. We also assessed changes in body weight.
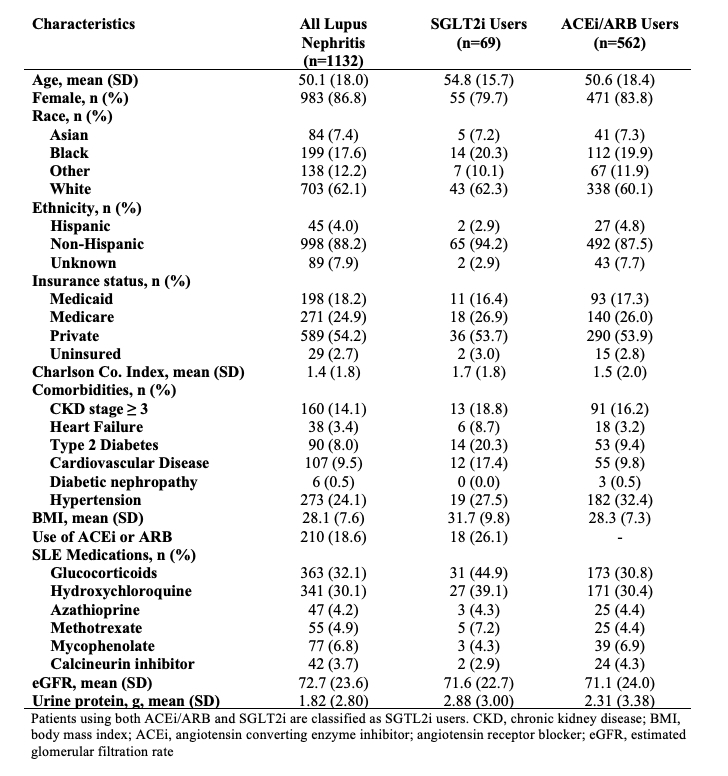
Results: Of 1416 patients with LN, 1132 met the primary eligibility criteria for SGLT2i use, 935 met the eligibility criteria with stricter GFR or proteinuria cutoffs, and 418 met eligibility criteria in the maintenance phase (Figure 1). Of those meeting the primary eligibility criteria, 69 (6%) initiated an SGLT2i and 562 (50%) initiated an ACEi/ARB (Table 1). The mean age of SGLT2i users was 55 years, vs. 50 years among all eligible patients with LN, and 80% were female vs 87% of eligible patients with LN were female. 20% of SGLT2i users were Black vs. 18% with LN. Comorbidities among SGLT2i users included 20% with type 2 diabetes, 9% with heart failure, and 19% with CKD stage ≥3. Adverse event rates were similar among SGLT2i initiators vs ACEi/ARB initiators with LN (Table 2). There were no cases of necrotizing fasciitis or DKA. SGLT2i users had mean weight loss of 1.9 kg vs. 0.5 kg for ACEi/ARB users (p=0.19).
Conclusion: In this clinical cohort of patients with LN and a potential indication for SGLT2i use, SGLT2i were infrequently used in recent years. Compared with ACEi/ARB users, SGLT2i users had similar rates of adverse events, including genitourinary infections and fractures. Further follow-up studies are needed to determine the impact of SGLT2i on long-term kidney outcomes in LN and to determine the optimal eligibility criteria for patients with LN.
To cite this abstract in AMA style:
Jorge A, Panchot K, Zhou B, Patel A, Choi H. Use and Safety of Sodium-Glucose Cotransporter-2 Inhibitors Among Patients with Lupus Nephritis and Clinical Indications [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/use-and-safety-of-sodium-glucose-cotransporter-2-inhibitors-among-patients-with-lupus-nephritis-and-clinical-indications/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/use-and-safety-of-sodium-glucose-cotransporter-2-inhibitors-among-patients-with-lupus-nephritis-and-clinical-indications/