Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: To expand treatment options, increase access to life-saving medications, and lower healthcare costs through competition, the US Congress created an abbreviated licensure pathway for biosimilars.1 Based upon scientific justification including: PK, PD, efficacy, safety, and immunogenicity data, two tumor necrosis factor inhibitor (TNFi) biosimilars were approved for all reference biologic indications (e.g., ankylosing spondylitis, psoriatic arthritis) despite clinical efficacy studies in just one indication (rheumatoid arthritis)a—a process known as extrapolation. Non-inferiority trial design for one approved indication and extrapolation based labeling for other indications represent potential barriers to physician adoption of biosimilars. This study aimed to assess community rheumatologists’ perceptions and utilization patterns of the two TNFi biosimilars approved and commercialized in the US.
Methods: A live meeting in November 2018 surveyed US-based community rheumatologists regarding their perceptions and utilization patterns of biosimilars. Participants completed a web-based premeeting survey and live queries captured via an audience response system. Physician characteristics and responses were summarized using descriptive statistics.
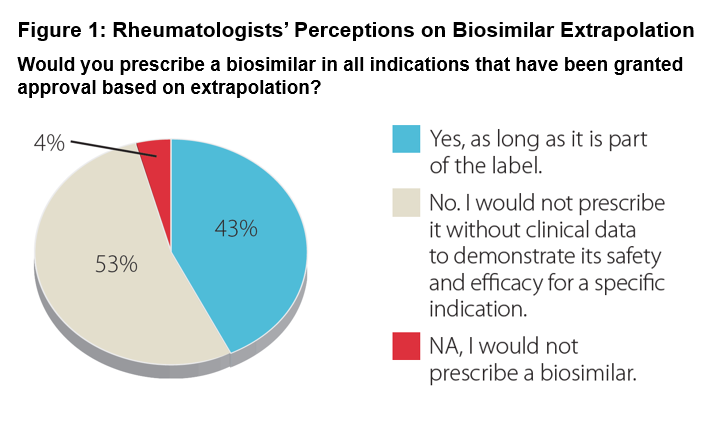
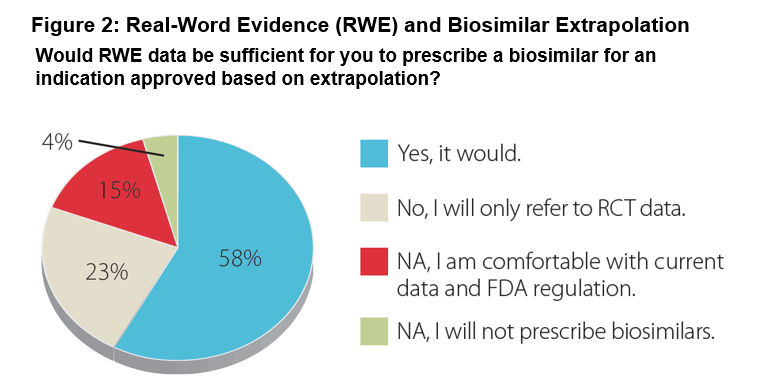
Results: 54 rheumatologists participated describing their practices as: urban 48%, suburban 45%, and rural 7%. Median years in practice were 19 years (range 3-40). 67% have prescribed biosimilars since being commercially available in late 2016. Regarding their familiarity with biosimilars and the FDA approval process: 34% very familiar, 56% somewhat familiar, and 10% not. A minority of the rheumatologists (15%) expressed comfort with current biosimilar safety/efficacy data and FDA regulation. Regarding extrapolation-based labeling: 53% said they would not prescribe without data for a specific indication (Figure 1). Regarding additional data: 58% indicated that real-world evidence would suffice for an extrapolated indication, while 23% would only accept randomized controlled trials (Figure 2). Participants stated they are most likely to prescribe a biosimilar when it provides cost-savings to the patient or their practice with 48% requiring a 31-50% discount from the reference product for them to consider a biosimilar over the reference biologic. Various concerns remain regarding prescribing a biosimilar, including FDA evaluation process and payer coverage and/or reimbursement for a biosimilar.
Conclusion: The majority of the participating rheumatologists appear to be familiar with biosimilars and its FDA approval process, and they are open to prescribing them, especially when there are cost-savings. Further safety/efficacy data is needed for rheumatologists to willingly expand their use of biosimilars to the indications that were approved based on extrapolation.
1FDA.gov
aOne of the TNFi biosimilars had an additional clinical study in Crohn’s disease.
To cite this abstract in AMA style:
Yeh T, Jeune-Smith Y, Phillips E, Gajra A, Feinberg B. US Community Rheumatologists’ Knowledge and Perceptions of Biosimilar Expanded Indication Approval by Extrapolation [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/us-community-rheumatologists-knowledge-and-perceptions-of-biosimilar-expanded-indication-approval-by-extrapolation/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/us-community-rheumatologists-knowledge-and-perceptions-of-biosimilar-expanded-indication-approval-by-extrapolation/