Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects multi-organ systems, particularly the kidneys. Urine is an ideal source of SLE biomarkers as it can be collected noninvasively. Urinary proteins, filtered from the blood or produced by kidney cells, can indicate both systemic and kidney-specific diseases. Posttranslational modifications (PTMs) of proteins, such as acetylation, can occur after protein synthesis and reflect disease states. Human serum albumin (HSA), the most abundant plasma protein, has been found to undergo acetylation at 25 lysine residues. This modification can alter the charge and isoelectric point of HSA. We aimed to identify urinary biomarkers of acetylated PTMs to detect kidney damage in patients with SLE.
Methods: A global proteomics analysis of urine samples from 11 healthy controls, 12 SLE patients, and 7 newly diagnosed lupus nephritis (LN) patients was conducted to screen for PTMs. This was followed by multiple reaction monitoring (MRM) analysis in 25 healthy subjects, 29 SLE patients, 32 newly diagnosed LN patients, and 16 treated LN patients to validate the findings.
Stable isotope-labeled internal standard (SIS) peptides for five acetylated peptides (albumin at K36, K161, and K402; serotransferrin at K588; and C11orf40 at K45) were synthesized. These transitions were optimized using a high-performance liquid chromatography (HPLC) system (1290 Infinity, Agilent Technologies) and triple quadrupole mass spectrometry (MS) with a jet stream Electrospray Ionization (ESI) source (6495 Agilent, Agilent Technologies). Five μg of urine peptide spiked with 25 fmol of 7 SIS peptides were analyzed via the MRM method. Peak areas of each peptide transition were extracted and normalized using the spiked heavy peptide. Peptide amounts were calculated using a standard curve and normalized by the creatinine value of each patient’s urine. Outliers exceeding six standard deviations were removed, and box plots and t-tests were generated using Prism (ver. 10.2.3).
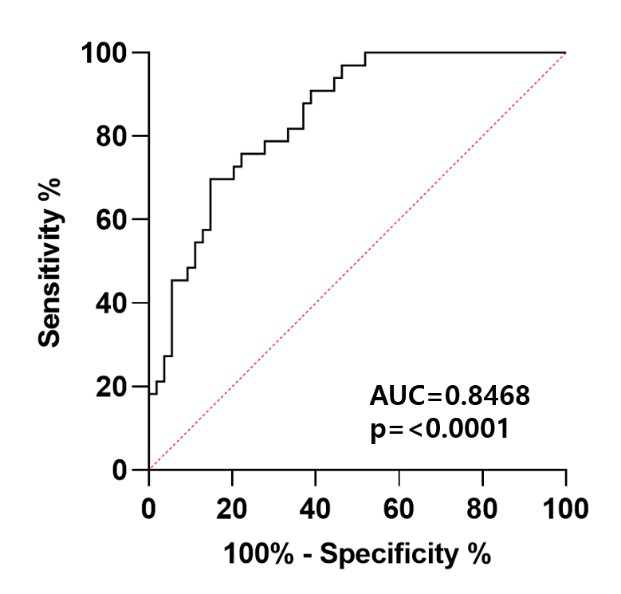
Results: A global proteomics analysis identified 15 proteins with acetylated lysine (K) peptides in urine samples, including albumin, serotransferrin, and C11orf40. Specifically, albumin had 34 acetylated lysine sites, with 7 sites (K36, K130, K161, K264, K305, K396, and K402) being at significantly higher concentrations in patients newly diagnosed with LN. Additionally, acetylated C11orf40 at K45 and serotransferrin at K588 levels were statistically significant. An MRM analysis confirmed that acetylation at albumin K402 was significantly higher in those with newly diagnosed LN compared to those with SLE, treated LN, and healthy controls and was correlated positively with proteinuria (p = 0.006). A receiver operating characteristic (ROC) analysis indicated that acetylated albumin at K402 is a highly accurate predictor of newly diagnosed lupus nephritis (AUC = 0.8468, p < 0.0001) (Figure 1).
Conclusion: Acetylated albumin at K402 is a novel potential urine biomarker for detecting renal damage in patients with newly diagnosed lupus nephritis.
To cite this abstract in AMA style:
Lee Y, Lee E, Kim M, Cho M, Ahn S, Hong S, Oh J, Lee C, Yoo B, Kim Y. Urinary Acetylated Albumin as a Biomarker of Nephritis in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/urinary-acetylated-albumin-as-a-biomarker-of-nephritis-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/urinary-acetylated-albumin-as-a-biomarker-of-nephritis-in-patients-with-systemic-lupus-erythematosus/