Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Metabolic & Crystal Arthropathies – Basic & Clinical Science (1897–1900)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Urate lowering therapy (ULT) is a cornerstone treatment in the management of gout. A paucity of data exists about the relative efficacy and safety of the two major oral ULTs, allopurinol and febuxostat, when administered as part of a treat-to-target approach as recommended by ACR and EULAR. Evidence regarding the comparative efficacy and safety of these agents is particularly needed in the context of chronic kidney disease (CKD), a common comorbid condition among gout patients. This multicenter, randomized, double-blind, non-inferiority trial was designed to examine the comparative efficacy and safety of these ULTs in gout management.
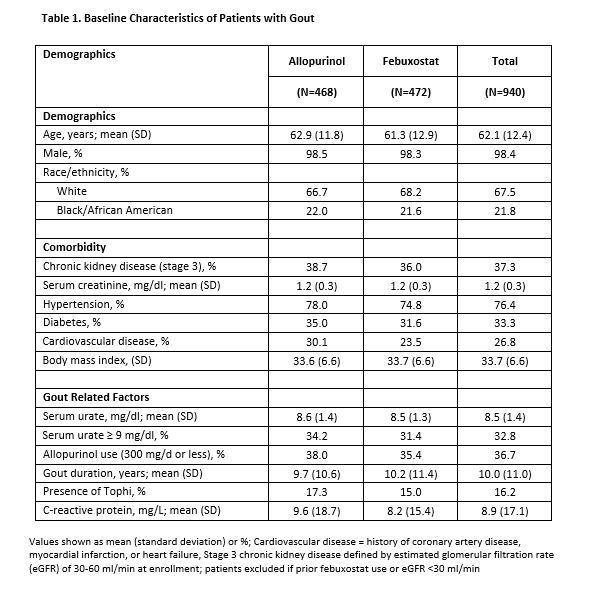
Methods: Patients with gout and a serum urate (SU) concentration ≥ 6.8 mg/dl were randomized 1:1 between 2017-2019 to receive appropriately titrated allopurinol or febuxostat in this non-inferiority 72-week trial. Patients with persistent hyperuricemia, despite treatment with allopurinol (≤ 300 mg/dl), were eligible and the protocol specified that ≥ 1/3 would have CKD stage 3. The trial had 3 phases: 1) ULT titration (weeks 0-24), 2) Maintenance (weeks 25-48), and 3) Observation, with continued stable ULT (weeks 49-72). Allopurinol and febuxostat were initiated in daily doses of 100 mg and 40 mg with maximum titration to 800 mg and 120 mg (reduced to 80 mg in 2019 per FDA’s request), respectively. Patients received anti-inflammatory prophylaxis chosen by site investigator according to 2012 ACR guidelines until the beginning of phase 3. The primary endpoint was the proportion of patients experiencing ≥ 1 flare during phase 3 with a pre-specified margin of < 8% difference indicative of non-inferiority. Secondary endpoints included: efficacy/tolerability in CKD stage 3, proportion achieving SU < 6 mg/dl at the end of phase 2, and serious adverse events (SAE).
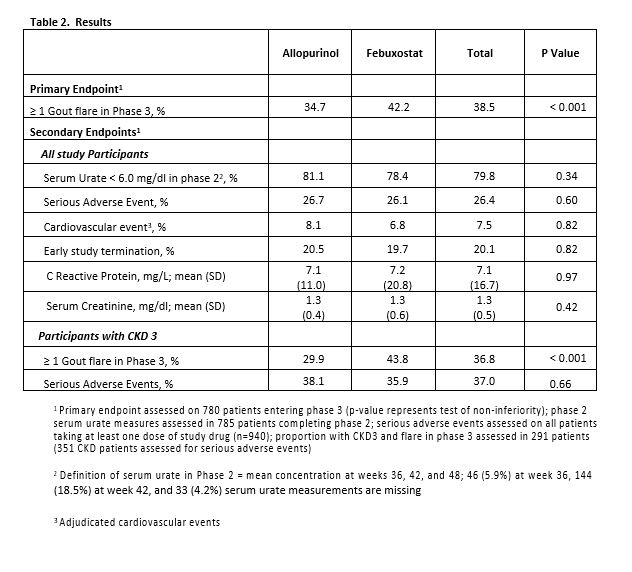
Results: : Characteristics of the 940 patients with gout (n=21 sites) receiving at least 1 dose of study medication are shown in Table 1. Overall, 20% withdrew prior to completion with similar proportions by treatment arm. During phase 3, 35% of patients treated with allopurinol had ≥ 1 flare compared to 42% of participants treated with febuxostat (p < 0.001 for non-inferiority) (Table 2). Overall, 80% of patients achieved SU < 6.0 mg/dl (92% achieved SU < 6.8 mg/dl) during phase 2 with no difference according to ULT treatment. Likewise, there were no treatment differences in SAEs (including percent of patients with cardiovascular events) in those with or without CKD. Additional secondary outcomes are shown in Table 2.
Conclusion: This large, randomized double-blind trial demonstrates that allopurinol, when dosed appropriately as part of a treat-to-target strategy, is non-inferior to febuxostat in the treatment of gout. Both ULTs were highly efficacious in this context with 80% of patients achieving and maintaining SU goals after 1 year and more than 90% achieving SU < 6.8 mg/dl. There was no evidence of increased cardiovascular toxicity with febuxostat compared to allopurinol. Moreover, the comparative efficacy and safety of these two agents extended to patients with CKD stage 3, highly relevant as nearly one in every two gout patients suffers from renal insufficiency.
To cite this abstract in AMA style:
O'Dell J, Neogi T, Pillinger M, Palevsky P, Newcomb J, Brophy M, Wu H, Davis-Karim A, Ferguson R, Pittman D, Terkeltaub R, Cannella A, England B, Helget L, Mikuls T, Taylor T. Urate Lowering Therapy in the Treatment of Gout: A Multicenter, Randomized, Double-blind Comparison of Allopurinol and Febuxostat Using a Treat-to-Target Strategy [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/urate-lowering-therapy-in-the-treatment-of-gout-a-multicenter-randomized-double-blind-comparison-of-allopurinol-and-febuxostat-using-a-treat-to-target-strategy/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/urate-lowering-therapy-in-the-treatment-of-gout-a-multicenter-randomized-double-blind-comparison-of-allopurinol-and-febuxostat-using-a-treat-to-target-strategy/