Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Upadacitinib (UPA) is an oral reversible JAK inhibitor engineered for greater selectivity for JAK1 vs JAK2, JAK3, and TYK2, and is currently being assessed for the treatment of RA. RAPID3 is a pooled index of the three key patient-reported measures: patient global assessment, pain, and physical function. In this analysis we assessed the effect of UPA treatment on RAPID3 in the SELECT-BEYOND, SELECT-COMPARE, and SELECT-MONOTHERAPY trials.
Methods: In SELECT-BEYOND1, bDMARD-IR pts received UPA 15 mg or 30 mg once daily (QD), or PBO for 12 wks while continuing stable csDMARD therapy. In SELECT-COMPARE2, MTX-IR pts received UPA 15 mg QD, PBO, or ADA 40 mg every 2 wks for 12 wks while continuing MTX. In SELECT-MONOTHERAPY3, pts received UPA monotherapy 15 mg or 30 mg QD, or continued MTX monotherapy (cMTX) for 14 wks. We assessed the least squares mean changes from baseline (BL) in RAPID3 and the proportion of pts reporting RAPID3 remission (≤3), low (LDA, >3 to ≤6), moderate (MDA, >6 to ≤12), and high disease activity (HDA, >12). Correlations between RAPID3 remission and remissions defined by CDAI, SDAI, and DAS28(CRP) were also assessed using Pearson correlation coefficients. Non-responder imputation was used for categorical endpoints, and last observation carried forward for continuous endpoints in pts who received rescue therapy in SELECT-COMPARE. Other continuous data are as observed. Data were analyzed descriptively.
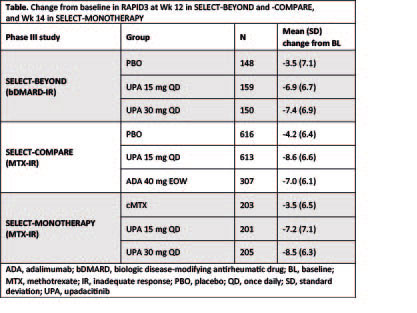
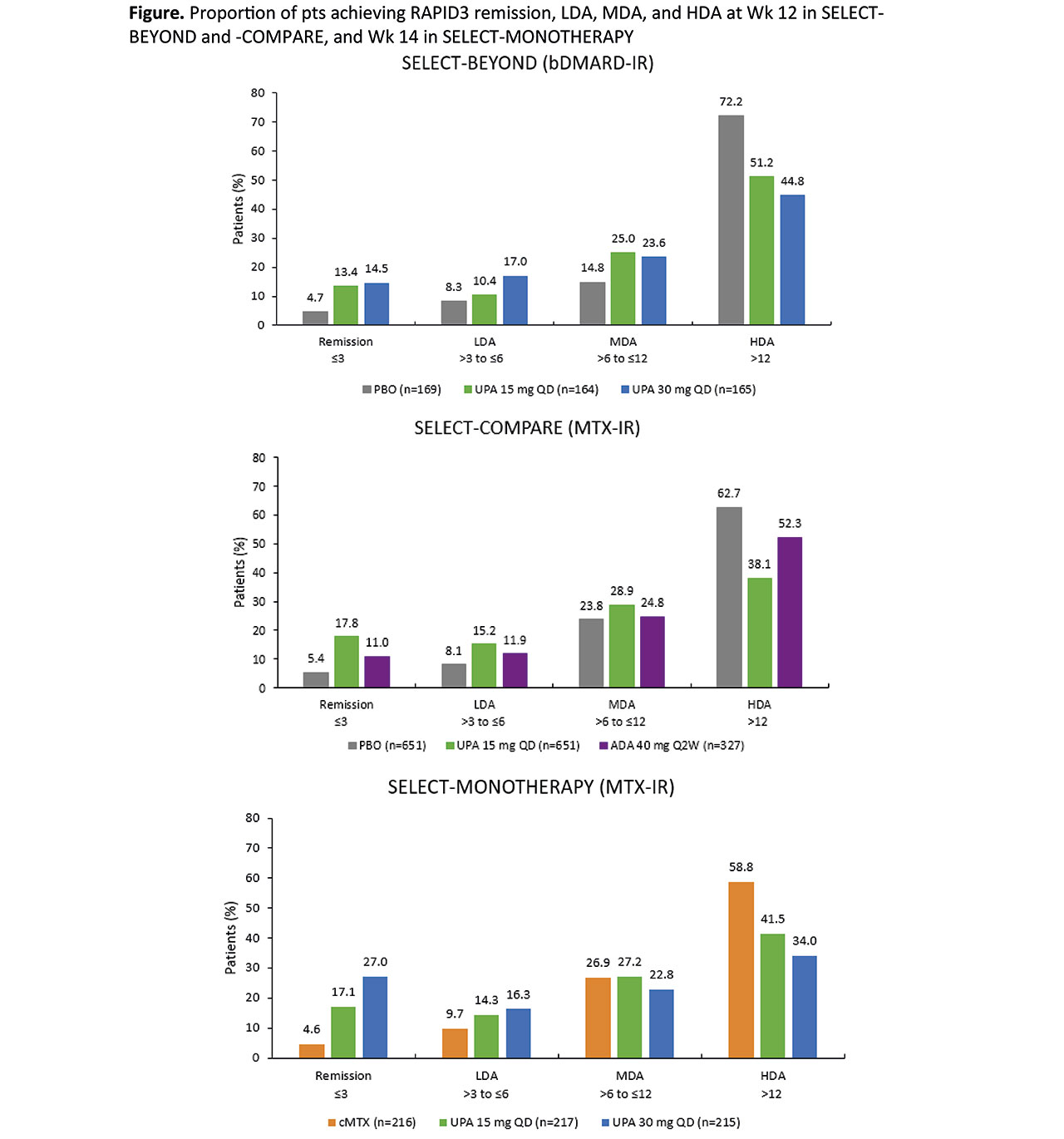
Results: 498, 648, and 1629 pts were randomized in SELECT-BEYOND, -MONOTHERAPY, and -COMPARE, respectively. Numerically higher improvements from BL in RAPID3 were reported with UPA 15 mg and 30 mg treatment vs PBO in SELECT-BEYOND, and vs cMTX in SELECT-MONOTHERAPY (Table). UPA 15 mg QD was also associated with greater reductions from baseline in RAPID3 vs PBO and ADA in SELECT-COMPARE (Table). Of note, the improvements in RAPID3 with UPA and ADA exceeded the minimal clinically important difference (MCID = 3.84). The proportions of pts achieving RAPID3 remission were numerically higher in the UPA 15 mg and 30 mg groups vs PBO and cMTX in SELECT-BEYOND and SELECT-MONOTHERAPY, respectively (Figure). In addition, the proportions of pts in RAPID3 HDA were lower with UPA vs PBO and cMTX in these trials. In SELECT-COMPARE, higher rates of RAPID3 remission, with fewer pts in HDA, were evident with UPA 15 mg treatment vs PBO and ADA (Figure). Correlations between RAPID3 and other remission endpoints were significant (p< 0.001; r=0.35–0.75) in all three trials at both baseline and Wk 12/14.
Conclusion: UPA was associated with improvements in RAPID3, both as monotherapy and in combination, in MTX-IR (SELECT-COMPARE and SELECT-MONOTHERAPY) or bDMARD-IR (SELECT-BEYOND) pts. UPA treatment can result in improved patient-reported disease activity, pain, and physical function in RA.
References
- Genovese MC, et al. Lancet 2018; 391:2513–24
- Fleischmann R, et al. Arthritis Rheumatol 2018;70(Suppl. 10): Abstract 890
- Smolen JS, et al. Arthritis Rheumatol 2018;70(Suppl. 10): Abstract 889
- Ward MM, et al. J Rheumatol 2019;46:27–30
To cite this abstract in AMA style:
Bergman M, Tanaka Y, Citera G, Bahlas S, Ali M, Meerwein S, Song Y, Strand V. Upadacitinib Treatment and the Routine Assessment of Patient Index Data 3 (RAPID3) Among Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/upadacitinib-treatment-and-the-routine-assessment-of-patient-index-data-3-rapid3-among-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-treatment-and-the-routine-assessment-of-patient-index-data-3-rapid3-among-patients-with-rheumatoid-arthritis/