Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To evaluate the efficacy and safety of upadacitinib (UPA) monotherapy vs MTX monotherapy over 5 yrs among MTX-naïve patients with moderately to severely active RA in the long-term extension (LTE) of the phase 3 SELECT-EARLY trial.
Methods: Patients were randomized 1:1:1 to receive once daily UPA 15 mg or 30 mg or MTX (titrated up to 20 mg/wk by wk 8).1 At wk 26, patients who did not achieve CDAI remission (≤2.8) with < 20% improvement from baseline in TJC or SJC received rescue therapy (addition of MTX to insufficient responders in the UPA groups and addition of UPA 15/30 mg [by re-randomization] to insufficient responders in the MTX group). For patients who did not achieve CDAI remission but had ≥20% improvement in TJC and SJC at wk 26, background RA medications were optimized while patients remained on their original study drug. Per protocol amendment, all patients receiving UPA 30 mg were switched to the approved 15 mg dose, with the earliest switch occurring at wk 108. Efficacy assessments were evaluated over 5 yrs and are reported as observed (AO) for patients who received continuous monotherapy with UPA 15/30 mg or MTX. Results at wk 260 are also reported by randomized group for all patients, applying NRI for patients who were rescued or discontinued, with nominal P values. Treatment-emergent adverse events (TEAEs) per 100 patient-yrs were summarized over 5 yrs for those receiving monotherapy.
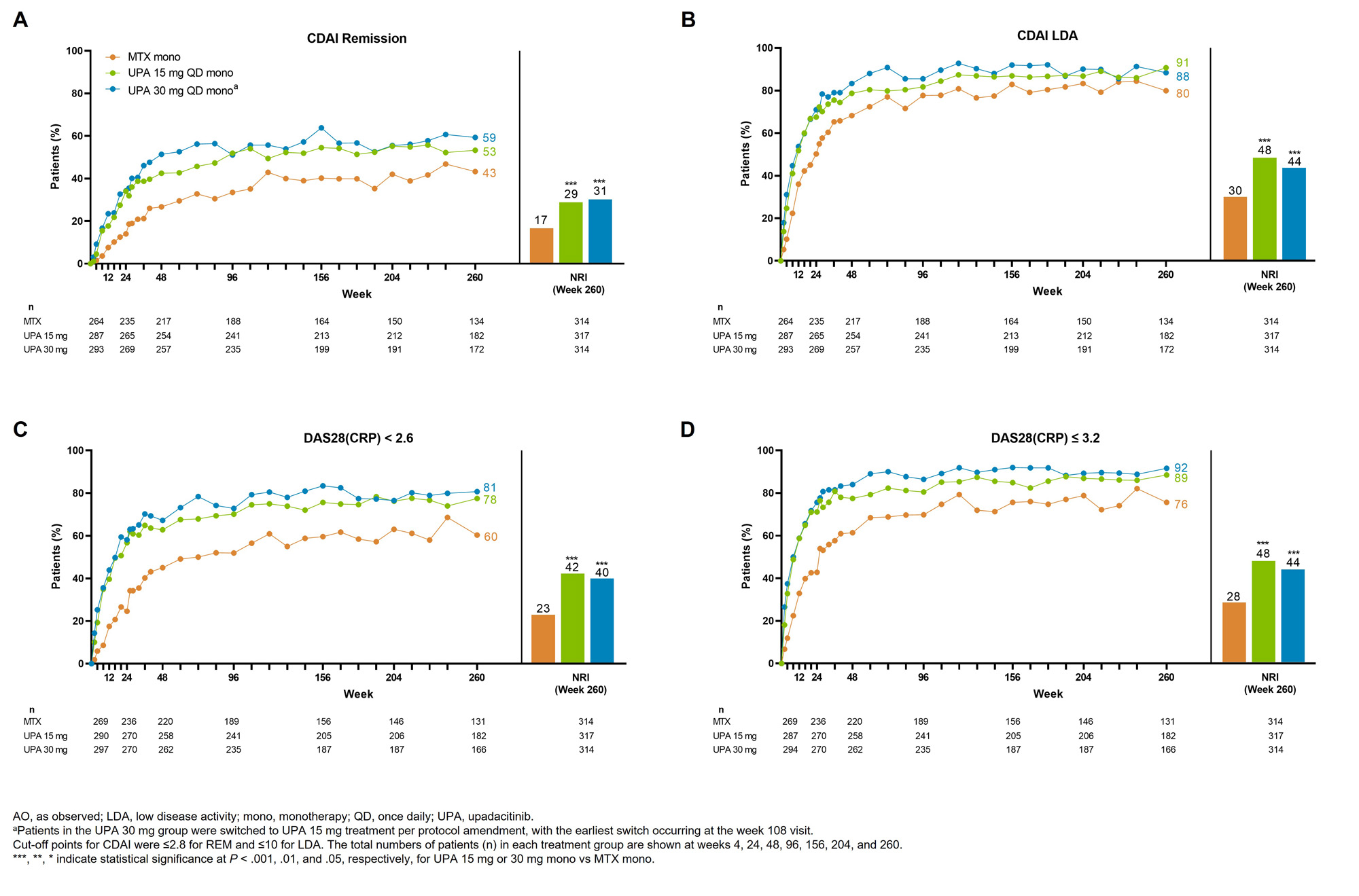
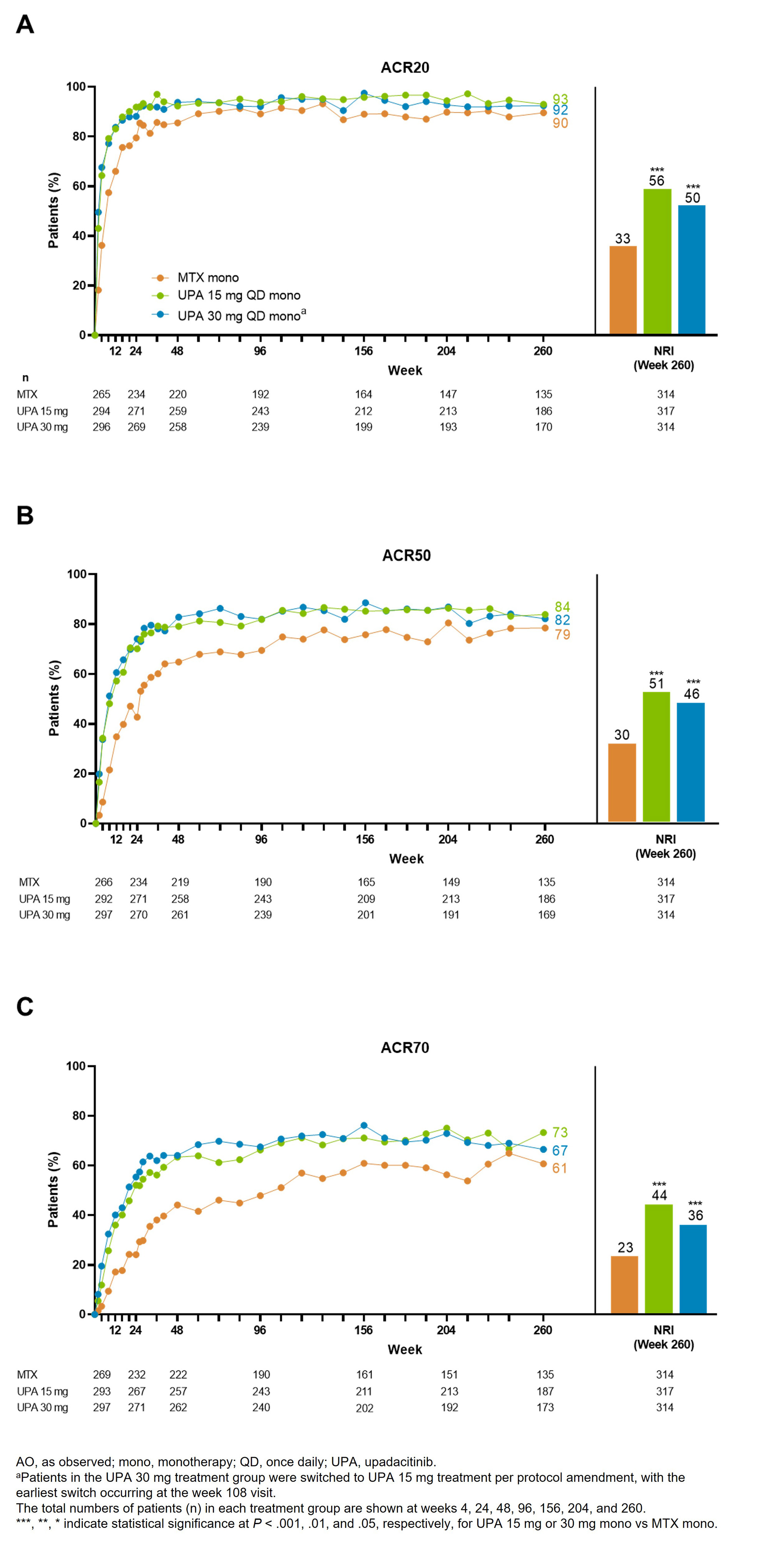
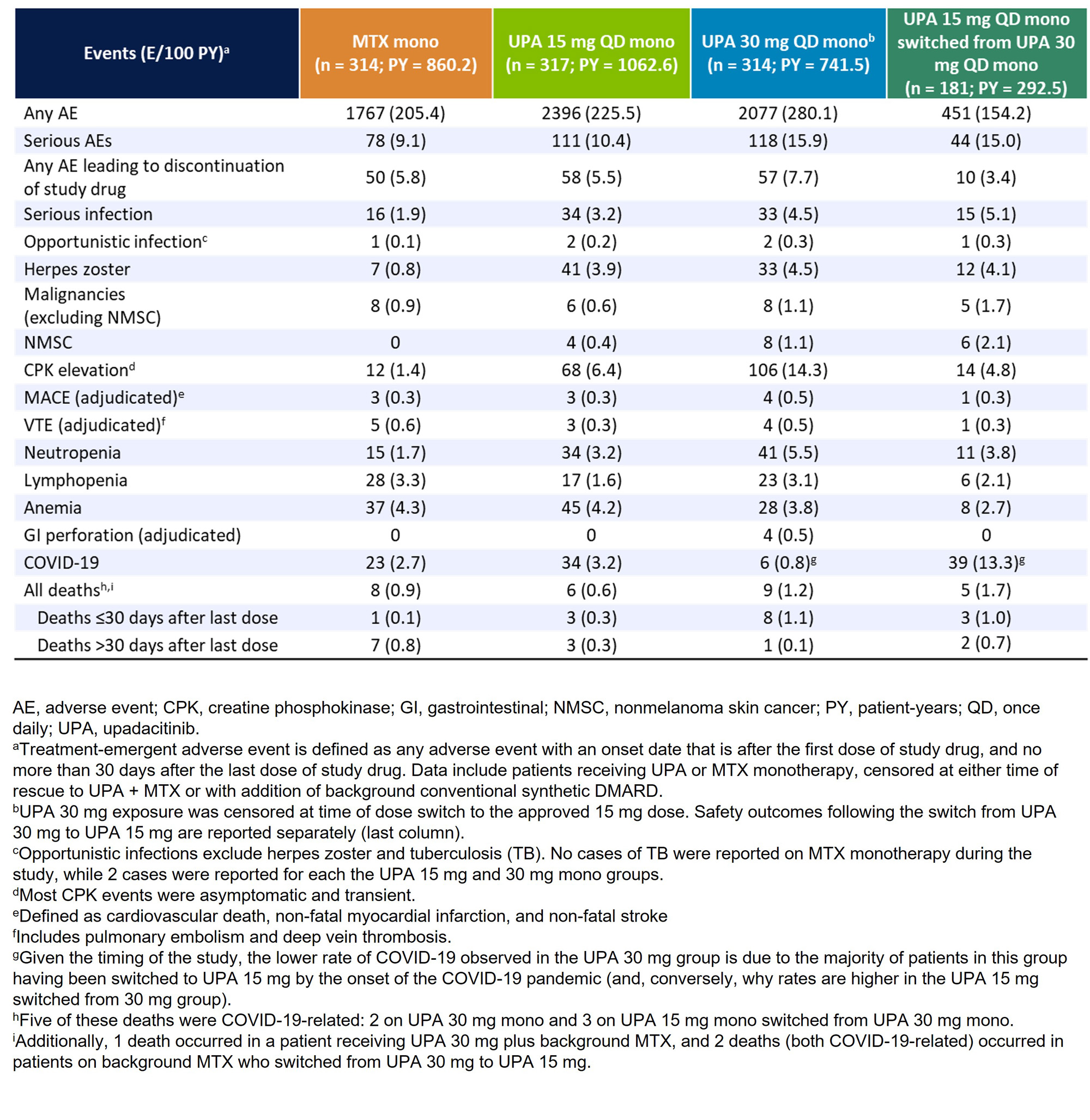
Results: Of 945 patients randomized and treated, 775 (82%) completed wk 48 and entered the LTE on study drug. Of these 775 patients, 255 (27%) discontinued study drug during the LTE due to the following primary reasons: TEAEs (9%), withdrawal of consent (7%), lack of efficacy (2%), lost to follow-up (3%), or other reasons (6%). Patients receiving UPA consistently demonstrated higher achievement of disease activity targets over 5 yrs compared with MTX (Figure 1, 2). In AO analyses, 53%/59% of patients attained CDAI remission with UPA 15/30 mg vs 43% on MTX at wk 260. NRI analyses also showed better CDAI, DAS28(CRP), and ACR/20/50/70 responses with UPA relative to MTX at wk 260 (nominal P < .001). Most TEAEs were numerically most frequent in patients receiving UPA 30 mg (Table). The rates of serious infections, herpes zoster, CPK elevation, nonmelanoma skin cancer (NMSC), and neutropenia were numerically higher with UPA than MTX. The overall rates of MACE, VTE, malignancy excluding NMSC, and lymphopenia were comparable across treatments. Rates of death were similar between UPA 15 mg and MTX but numerically higher with UPA 30 mg. Overall, the observed safety profile of UPA over 5 yrs was consistent with earlier results from this trial and the integrated phase 3 safety analysis.1-3
Conclusion: UPA 15 mg or 30 mg showed better clinical responses vs MTX in patients with RA throughout the 5-yr trial. Higher rates of several AEs, including serious infection, herpes zoster, and CPK elevation were observed with UPA compared to MTX. When used as monotherapy in MTX-naïve patients, UPA has better long-term efficacy vs MTX and a favorable benefit-risk profile.
References:
1. van Vollenhoven, R et al. Arthritis Rheumatol 2020; 72:1607–20
2. van Vollenhoven, R et al. Ann Rheum Dis2021; 80:568–59.
3. Cohen, SB et al. Ann Rheum Dis 2021;80: 304–11.
To cite this abstract in AMA style:
van Vollenhoven R, Strand V, Takeuchi T, Chávez N, Mannucci Walter P, Singhal A, Swierkot J, Khan N, Bu X, Li Y, Penn S, Camp H, Aelion J. Upadacitinib Monotherapy versus Methotrexate in Patients with Rheumatoid Arthritis: Efficacy and Safety Through 5 Years in the SELECT-EARLY Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/upadacitinib-monotherapy-versus-methotrexate-in-patients-with-rheumatoid-arthritis-efficacy-and-safety-through-5-years-in-the-select-early-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-monotherapy-versus-methotrexate-in-patients-with-rheumatoid-arthritis-efficacy-and-safety-through-5-years-in-the-select-early-randomized-controlled-trial/