Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients (pts) with active rheumatoid arthritis (RA) and inadequate response or intolerance to bDMARDs, treatment with upadacitinib (UPA), a JAK1-selective inhibitor resulted in significant improvements over 24 weeks (wks).1
We assessed UPA safety and efficacy through Wk60 in an ongoing extension of the phase 3 SELECT-BEYOND study.
Methods: SELECT-BEYOND enrolled a population of patients with active RA who had failed at least one prior biologic therapy.1 Pts received UPA 15mg or 30mg once daily (QD) or placebo (PBO) on top of background csDMARD treatment for 12 wks. From Wk12, pts randomized to UPA at baseline (BL) continued their assigned doses, while pts initially randomized to PBO received UPA 15mg or 30mg QD per pre-specified assignment at BL. Patients who completed Wk 24 entered the blinded long-term extension. Dose adjustments to UPA were not allowed. Adverse events (AE) per 100 pt years (PY) are summarized based on a cut-off date of 16 April 2018. Efficacy data up to the Wk60 visit are reported “As Observed”.
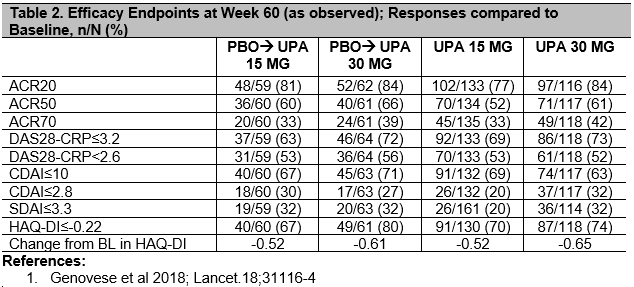
Results: 418/498 (84%) pts were randomized, completed 24 wks and entered the extension on study drug. By the safety data cut-off date, 19% pts discontinued study drug: 5% due to AE, 4% due to lack of efficacy, 3% withdrew consent, 2% were lost to follow-up, and 5% discontinued due to other reasons. Cumulative exposures to UPA15 and UPA30 were 301.7 and 290.7 PYs, respectively. Rates (Events/100PYs) of treatment-emergent AEs are reported (Table 1), and were numerically higher in the UPA30 vs UPA15 arm for serious AEs, AEs leading to discontinuation, serious infections, herpes zoster and hepatic disorders. Based on As Observed analysis, for pts completing Wk60 on UPA15 [172/216 (80%)] and UPA30 [168/202 (83%)], clinical and functional outcomes continued to improve compared to Baseline, or were maintained from Wk24 onwards1 in pts initially randomized to UPA15 or 30; Remission by CDAI≤2.8 at Wk60 was achieved by 20% and 32%, respectively, and DAS28-CRP< 2.6 was achieved by 53% and 52%. Pts who were switched to UPA from PBO at Wk12 had comparable efficacy to pts initially randomized to UPA (Table 2).
Conclusion: The benefit:risk of upadacitinib treatment in this refractory population remains favorable. No new safety signals were identified. Some AEs were numerically higher for UPA30 vs 15; however the clinical significance of this, the assessment of rare safety events in this study, and the overall benefit:risk of upadacitinib 15mg and 30mg in the treatment of RA are best evaluated in an integrated analysis across the phase 3 program. UPA15mg and 30mg continued to be effective in treating RA signs and symptoms, and in improving physical function.
To cite this abstract in AMA style:
Genovese M, Combe B, Hall S, Rubbert-Roth A, Zhong S, Meerwein S, Pangan A, Fleischmann R. Upadacitinib in Patients with Rheumatoid Arthritis and Inadequate Response or Intolerance to Biological DMARDs: Results at 60 Weeks [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/upadacitinib-in-patients-with-rheumatoid-arthritis-and-inadequate-response-or-intolerance-to-biological-dmards-results-at-60-weeks/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-in-patients-with-rheumatoid-arthritis-and-inadequate-response-or-intolerance-to-biological-dmards-results-at-60-weeks/