Session Information
Date: Saturday, November 16, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is one of the most severe complications in idiopathic inflammatory myopathies (IIM), substantially contributing to the morbidity and mortality. ILD associated with IIM (IIM-ILD) is usually treated with glucocorticoids in combination with tacrolimus and/or cyclophosphamide, or tofacitinib, or other immunosuppressants. However, the outcomes of patients in some subgroups of IIM-ILD remain unsatisfactory and the management of IIM-ILD is a challenging filed under active investigation. Thus, upadacitinib (UPA), a selective JAK1 inhibitor, is used in the treatment of IIM-ILD patients who have failed the first-line therapies.
Methods: Patients with IIM-ILD managed from Dec, 2022 to May, 2024 in the second affiliated hospital, Zhejiang University School of Medicine (SAHZU) were reviewed, and those treated with UPA were identified. The clinical characteristics, pulmonary function test (PFT), computed tomography (CT), and medications both at baseline and after treatment were collected and analyzed.
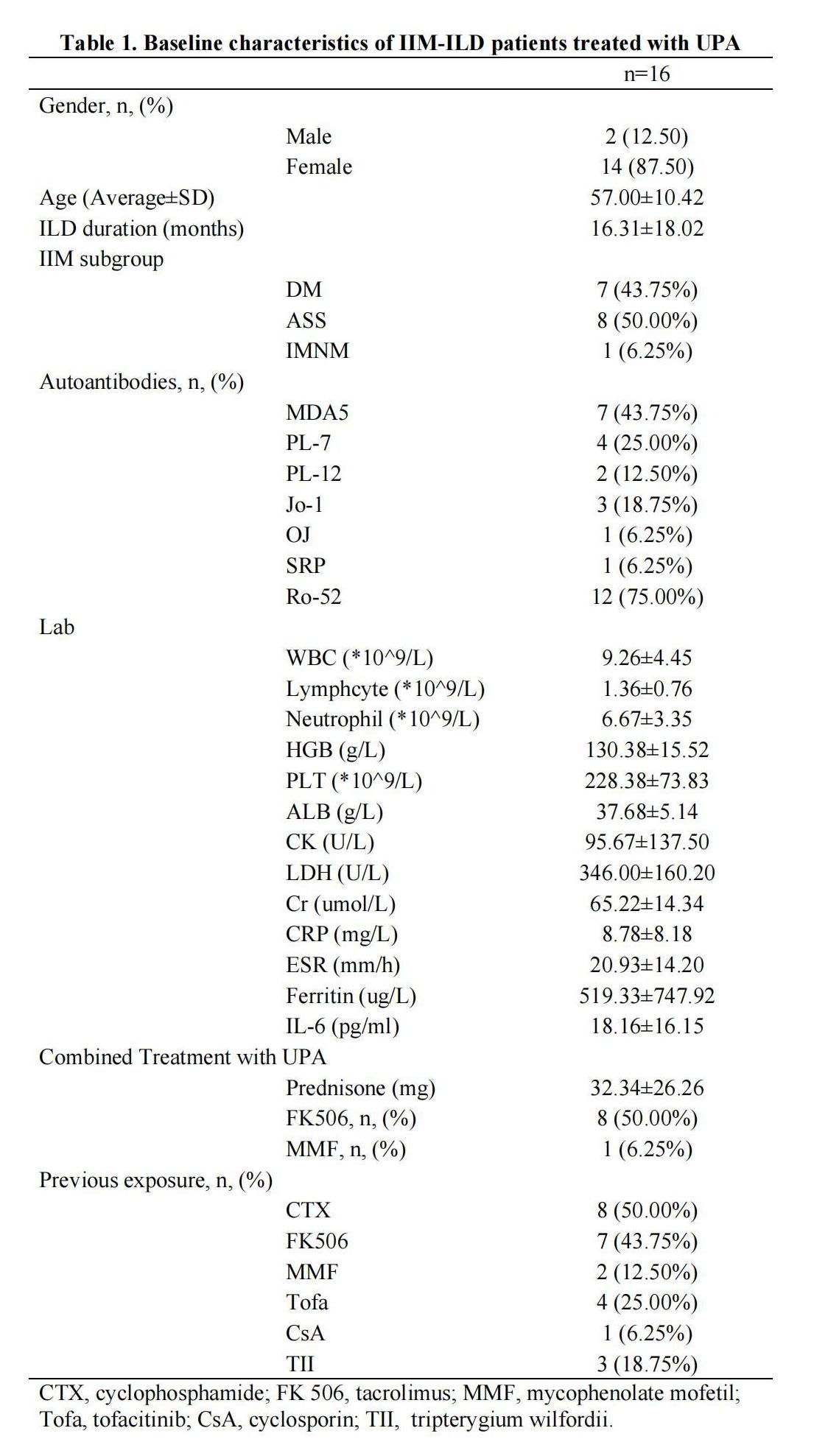
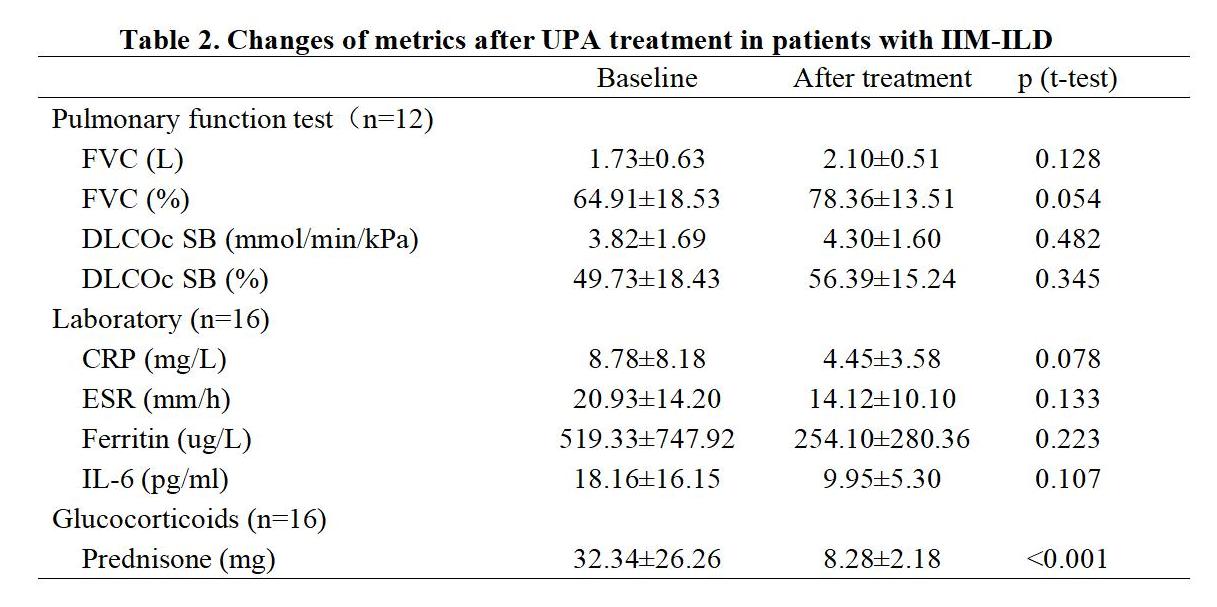
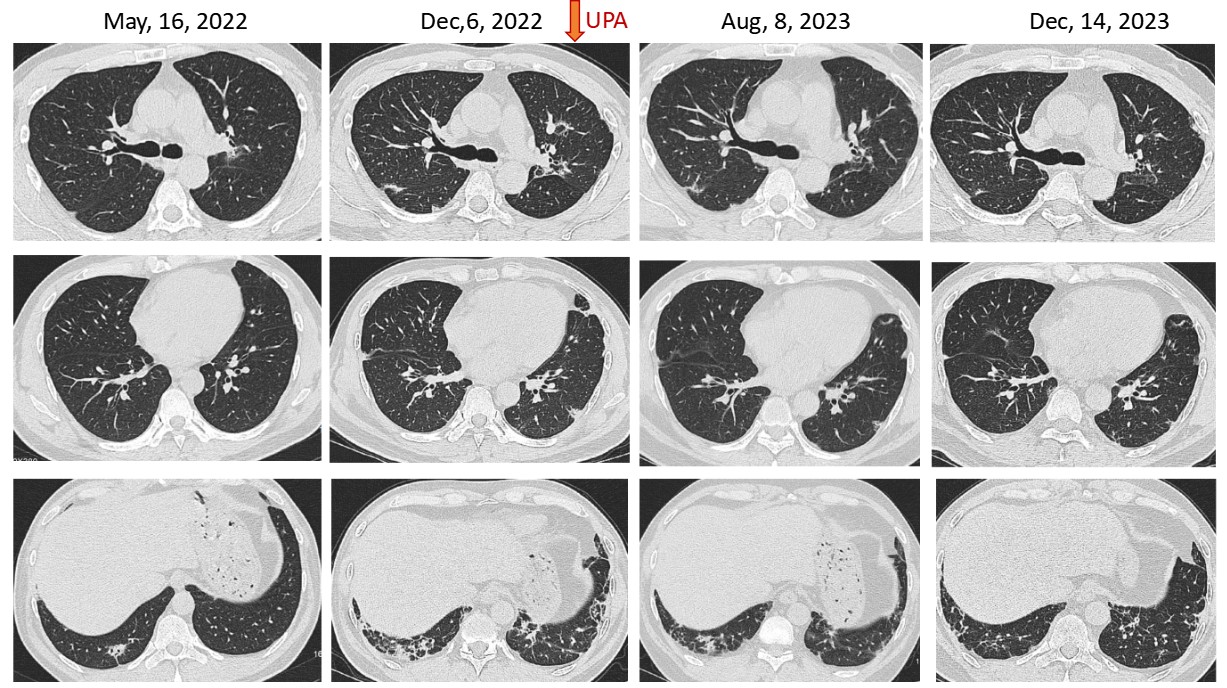
Results: A total of 28 IIM-ILD patients were treated with UPA, with 16 having PFT or CT at baseline and after treatment of more than 3 months. The average age of these 16 patients was 57.00±10.42 years, and 14 (87.50%) were female. Eight (50.00%) patients were diagnosed with anti-synthetase syndrome, seven (43.75%) with dermatomyositis, and one (6.25%) with immune-mediated necrotizing myopathy. The anti-myositis specific antibodies identified in these patients included anti-MDA5, anti-PL7, anti-Jo-1, anti-PL-12, anti-OJ, and anti-SRP, in the order of decreased frequency. Twelve (75.00%) patients were anti-Ro-52 positive. Previous therapeutic exposures before the application of UPA included cyclophosphamide (50.00%), tacrolimus (43.75%), tofacitinib (25.00%), Tripterygium wilfordii (18.75%), mycophenolate mofetil (12.50%), and cyclosporin (6.25%). At the time of UPA initiation, all patients were on corticosteroids with an average dose of 32.34±26.26mg prednisone or its equivalent, and eight (50.00%) patients were treated in combination with tacrolimus and one (6.25%) with mycophenolate mofetil (Table 1). These UPA treated IIM-ILD patients were followed up for 7.42 ±3.75 months, and the absolute FVC, FVC%, absolute DLCOc SB, and DLCOc SB% were all increased after treatment. Although, no significant difference was observed comparing the baseline PFTs with those after treatment, the FVC% increased from 64.91±18.53% at baseline to 78.36±13.51% after treatment (p=0.054) (Table 2). In several patients, the pulmonary infiltrates were apparently decreased (Figure 1). Moreover, the dosage of glucocorticoids significantly decreased after treatment with UPA (p< 0.001). Among the 28 IIM-ILD patients treated with UPA, six experienced infections and discontinued UPA, with two diagnosed with COVID-19, one with pneumocystis jiroveci pneumonia, one with herps zoster, one with lymphangitis, and one with unknown etiology.
Conclusion: UPA appeared as a considerable option in the treatment of IIM-ILD. The effectiveness and safety warrant further validation in larger cohorts.
To cite this abstract in AMA style:
Zhang T, Wang Q, Sun W, Liu L, Xue J. Upadacitinib in Interstitial Lung Disease Associated with Idiopathic Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/upadacitinib-in-interstitial-lung-disease-associated-with-idiopathic-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/upadacitinib-in-interstitial-lung-disease-associated-with-idiopathic-inflammatory-myopathies/