Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: VEXAS syndrome is an adult-onset, X-linked, life-threatening, autoinflammatory and hematological disease caused by somatic mutation in UBA1 gene. To understand its pathophysiology, we aimed to achieve a molecular and phenotypic characterization of hematopoiesis of VEXAS patients and to develop cellular and humanized mouse models by gene editing.
Methods: Six VEXAS patients (p.Met41 >Thr; p.Met41 >Val; p.Met41 >Leu; c.118-1 G >C) were recruited from our Unit. Variant allele frequency (VAF) of UBA1 mutant cells was quantified by targeted sequencing in isolated hematopoietic lineages and hematopoietic stem/progenitor cells (HSPCs). Multiparametric immunophenotypic analysis was performed on peripheral blood and bone marrow (BM), focusing on HSPCs. Circulating monocytes were analyzed by whole RNA-seq and metabolome analysis. Healthy age and sex-matched controls were included. To introduce UBA1 mutations and develop VEXAS models, cutting-edge gene editing technologies were adopted in healthy human HSPCs.
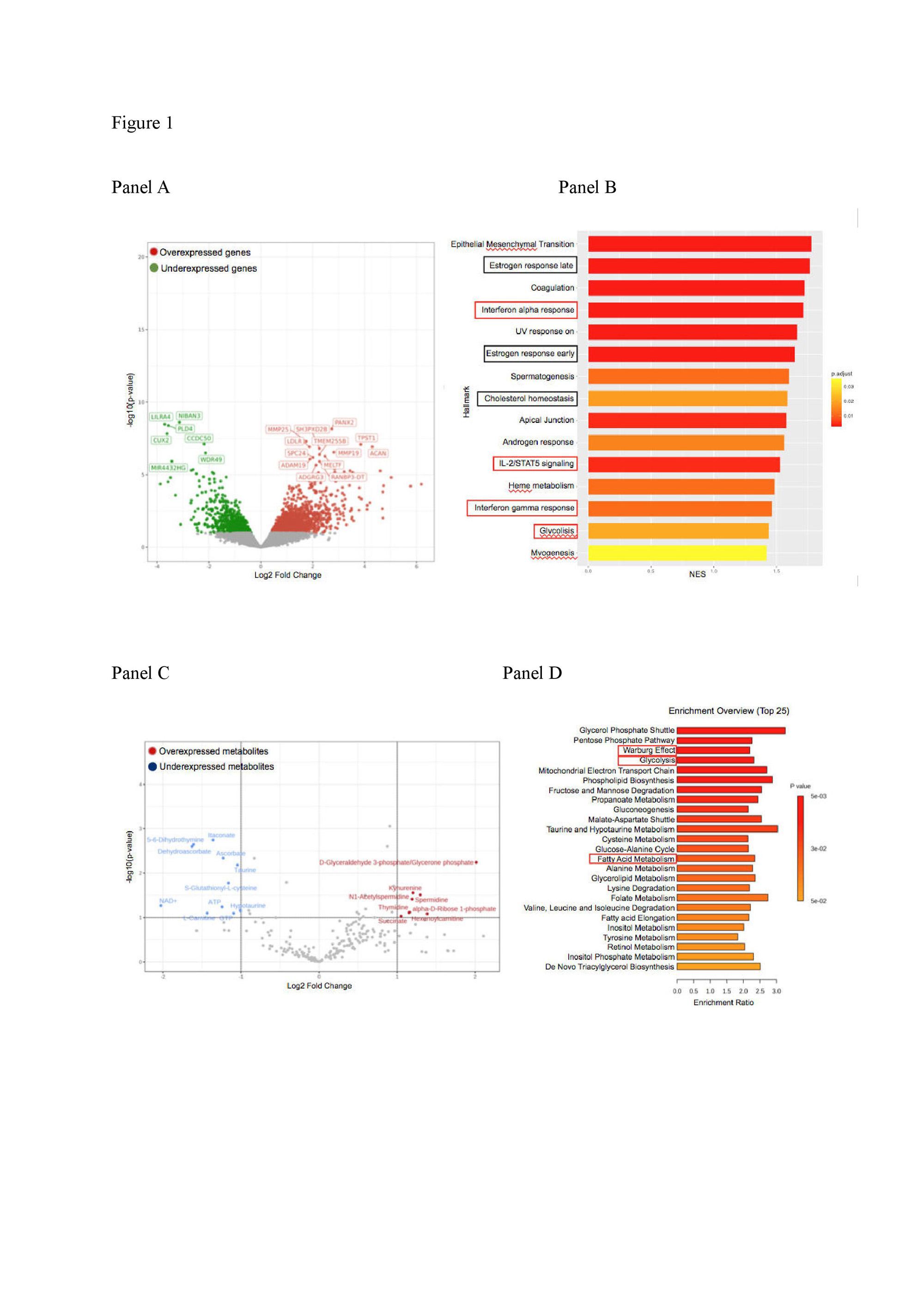
Results: Targeted sequencing in VEXAS patients showed >0.8 VAF in HSPCs. Conversely, VAF largely differed across mature cells, averaging 0.81 in neutrophils, 0.64 in monocytes, 0.42 in NK, 0.07 in T cells, and 0.09 in B cells, supporting a myeloid skewing of mutant HSPCs. Multiparametric immunophenotypic analyses showed unbalanced composition of HSPCs in the BM, with 2-to-3-fold reduction of primitive stem cells, multipotent and lymphoid progenitors, and 2-fold increase of myeloid progenitors, compared to matched healthy individuals. HSPCs, myeloid-biased HSPCs and immature myeloid cells were increased by 3-to-4 fold in the circulation (p< 0.03). Gene expression analysis of circulating monocytes displayed upregulation of inflammatory pathways and metabolic rewiring (Fig. 1, panels A-B). Metabolomic analyses confirmed hyperactivation of the glycolytic pathway and specific lipid metabolism (Fig. 1, C-D). Models of VEXAS generated by gene editing recapitulated patients’ hematopoiesis and pathophysiology in vitro and in vivo. UBA1 mutations were installed at VAF >0.9 in HSPCs and generated a myeloid bias in vitro. Transplantation of edited HSPCs in immunodeficient mice resulted in a 100-fold reduction in circulating B cells, while NK and myeloid compartments were preserved. Human BM HSPCs were 5-fold lower than control mice, largely myeloid-biased and presented abnormal vacuolar morphology. Concordantly, VAF was >0.8 in myeloid cells and HSPCs and < 0.3 in B cells.
Conclusion: Mutations in UBA1 drive expansion of HSPCs and enhance myelopoiesis-guided accumulation of myeloid precursors. Mutant lymphoid cells are negatively selected and their myeloid counterpart in peripheral blood displays upregulation of transcriptomic signatures and metabolic pathways indicative of inflammatory activation. Gene editing-based models hold promise to enable preclinical testing and validation of novel therapeutics to treat VEXAS syndrome.
To cite this abstract in AMA style:
Campochiaro C, Molteni R, Fiumara M, Tomelleri A, Diral E, Stefanoni D, Varesi A, Weber A, Alfieri R, Albano L, Panigada M, Cantoni E, Canarutto D, Basso-Ricci L, Quaranta P, D’Alessandro A, Matucci Cerinic m, Di Micco R, Scala S, Aiuti A, Ciceri F, Merelli I, Dagna L, Cenci S, Naldini L, Ferrari S, Cavalli G. Unraveling Pathophysiology and Hematopoiesis of VEXAS Syndrome by Multi-omics Analysis and Targeted Gene Editing [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/unraveling-pathophysiology-and-hematopoiesis-of-vexas-syndrome-by-multi-omics-analysis-and-targeted-gene-editing/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/unraveling-pathophysiology-and-hematopoiesis-of-vexas-syndrome-by-multi-omics-analysis-and-targeted-gene-editing/